Assessing Limits to Evolution and to Natural Selection: Reviews of Michael Behe’s “Edge of Evolution” and John Sanford’s “Genetic Entropy”
PREFACE (for blog) to Letter “STAN 4”: This is basically a continuation of “STAN 3.” It delves deeper into the mechanisms of evolution. It focuses on the issues raised by two anti-macroevolution books which Stan had cited. Michael Behe is perhaps best known for his 1996 book, Darwin’s Black Box, where he claimed that some complex biological features like the bacterial flagellum were “irreducibly complex.” In “The Edge of Evolution”(2007), Behe claims to have identified limits on the genetic changes that can be reasonably expected from random mutations and natural selection. Although his case is crippled by key mistakes in his thinking, Behe seems to make a sincere effort to engage real world data. In “Genetic Entropy,” John Sanford attempts to discredit mutations as a source of useful genetic change. In addition, he claims that, even if there were significant beneficial mutations, natural selection is not powerful enough to keep genomes from deteriorating. When I first read this book it seemed convincing, but as I dug into the literature I found the book misrepresents many issues. It is an interesting work from a psychological point of view, showing how the drive to reduce cognitive dissonance is so strong that people can be literally unable to see the facts that disagree with their viewpoint.
I hope that this compilation of literature results on various evolutionary topics will be useful to readers beyond the narrow creation/evolution debate. The contents are:
MICHAEL BEHE’S EDGE OF EVOLUTION
(1) Behe’s Fundamental Error
(2) Adaptive Responses to Environmental Changes
(3) Further Critiques of Edge of Evolution
(4) Status of Irreducible Complexity
(5) Behe and Intelligent Design
JOHN SANFORD’S GENETIC ENTROPY AND THE MYSTERY OF THE GENOME
(1) Kimura’s Distribution of Mutations
(2) Evidence for Beneficial Mutations
(3) Gene Duplication
(4) Natural Selection: What Sanford Claims
(5) Natural Selection: What Studies Show
Stability of Microbial Genomes
Mutation Accumulation Experiments With Eukaryotes
Fitness Recovery Experiments
(6) Evidence for Genomic Deterioration
James Crow’s Speculation on Human Genetic Deterioration
(7) Synergistic Epistasis and Other Theoretical Considerations
Effects of Synergistic Epistasis
Evidence for Synergistic Epistasis
Sanford’s Treatment of Synergistic Epistasis
Other Issues in Population Genetics
(8) CLOSING THOUGHTS
Bible Interpretation as the Fundamental Issue
*************************************************************
Dear Stan, December, 20XX
As part of our ongoing correspondence on the credibility of evolution, in my letter in July I offered the following critical comments on Michael Behe’s Edge of Evolution and John Sanford’s Genetic Entropy and the Mystery of the Genome:
Behe’s Edge of Evolution is a generally scholarly work. However, it makes a fundamental mistake which vitiates its entire thesis: it confuses the probability of obtaining one specific pair of point mutations (such as the pair that confer antibiotic resistance on the malaria parasite) with the probability of obtaining any possible pair of mutations anywhere in the genome. Thus, no edge to evolution was demonstrated.
Sanford’s Genetic Entropy, on the other hand, is simply wrong from beginning to end. It misrepresents everything it touches: beneficial and deleterious mutations, gene duplication, natural selection, and synergistic epistasis. In all these areas, Sanford avoids engaging the large body of work which directly refutes his viewpoint, and instead cherry-picks a few references that seem to point his way, usually misinterpreting them in the process.
Since these works are influential in some creationist circles and seem significant to you, I will go into more detail to substantiate those comments, with a focus on the efficacy of natural selection.
****************
MICHAEL BEHE’S EDGE OF EVOLUTION
The antimalarial drug chloroquine was introduced roughly 50 years ago. Since then, several independent strains have appeared of the malaria plasmodium P. falsiparum which are resistant to this drug. This resistance corresponds to two specific mutations, which alter two amino acids in a protein pump called PfCRT. From the total number of malaria organisms estimated to have lived over the past 50 years, and the number of times that resistance to the anti-malarial drug chloroquine has appeared in that period, Behe in Edge of Evolution estimates that the odds of chloroquine resistance appearance per malaria to be 10**-20. Behe then assumes (erroneously) that the odds of appearance of any significant change in protein binding are likewise around 10**-20 per individual. Since the number of all humans or all primates that ever lived is far smaller than 10**20, he concludes that there is no reasonable probability that even a single new protein binding site could have evolved in the hominid lineage [1]:
On average, for humans to achieve a mutation like this by chance, we would need to wait a hundred million times ten million years. Since that is many times the age of the universe, it’s reasonable to conclude the following: No mutation that is of the same complexity as chloroquine resistance in malaria arose by Darwinian evolution in the line leading to humans in the past ten million years.
For the moment, we will assume that Behe is correct that resistance to the anti-malarial drug chloroquine derives from two simultaneous, independent point mutations in genome of the malaria plasomodium P. falsiparum. If the rate of mutation at any one point is say 10**-8, then the probability of two specified mutations would be 10**-16. But the odds are low for any one such dual mutation getting broadly fixed in a population, partly because of the complicated life-cycle of the malaria plasmodium. If these odds are say 10**-4, then the probability of fixed appearance of chloroquine resistance per malaria plasmodium would be around 10**-20. Thus, standard evolutionary calculations yield a probability similar to Behe’s for the fixation of two independent point mutations.
(1) Behe’s Fundamental Error
So Behe may be right about the probability of chloroquine resistance. However, that says nothing about an “edge” to evolution. Although for chloroquine resistance in malaria there may be only one, narrow target (the two specific proteins in PfCRT), there are many, many other possible pairs of mutations in any living being. For organisms with say 10**9 base pairs, there are 10**18 possible two-point simultaneous mutations. Behe is implicitly assuming that only a single, pre-specified one of all these 10**18 pairs of mutations could possibly be useful in developing any new protein binding site. That is nonsense. Granted, most of these 10**18 pairwise mutations will be neutral or deleterious to fitness, and perhaps (as in the case of chloroquine resistance in malaria) there is only one pairwise mutation will achieve one specified result, but this in no way implies that some other pairwise mutation cannot achieve some other beneficial effect. As one reviewer noted, this is a “painfully basic” error on Behe’s part.
This error alone invalidates the thesis of Edge of Evolution. However, it gets even worse: Behe limits his consideration to single point mutations, and ignores the whole realm of frameshifts and duplications and insertions of large chunks of genetic material. He also fails to appreciate the sequential beneficial nature of many dual mutations, which can be crucial in enhancing the probability of their fixation. If the first of two mutations provides some advantage which promotes its fixation, then the odds of obtaining both mutations goes up considerably.
The insertion or deletion of a single nucleotide in the genome can cause the whole three-at-a-time translation of DNA into amino acids to shift. This frameshift mutation can immediately make a radically new protein – – no need to wait for 2 or 3 or 4 point mutations to occur [101] . As is usual for major mutations, most frameshift mutations are very deleterious to the organism. This is not a problem for evolution, since the highly deleterious mutations will be removed from the population by natural selection, whereas the rare beneficial frameshift mutation can give its bearer a selective advantage. ( For the record, the well-known “nylon bug” mutation, where a bacterium in a pond near a nylon plant in Japan acquired the novel ability to digest nylon-type molecules was originally thought to be a frameshift [2], but more recent work indicates it was a two amino acid substitution. [3] )
Behe claims that a gene modification requiring say 4 amino acid substitutions is so very improbable that it is way beyond the “edge of evolution.” Simply impossible. Yet Dennis Venema at Biologos [102] discusses studies done by Richard Lenki’s group on the evolution of a gene in the bacteriophage lambda which enables the binding of a second protein, and thus is unequivocally a gain-of-function. As it turns out, this mutation is exactly what Behe claims to be impossible: it involves at least 4 amino acid substitutions which are sequential (the new binding functionality does not appear until the fourth mutation is completed) , and which can be “found” (via many different mutational paths) by the phage in repeated experiments. So Behe’s conceptualization does not hold up in the real world.
(2) Adaptive Responses to Environmental Changes
Behe characterizes the response of the malaria to chloroquine as “trench warfare,” which diminishes the overall vigor of the malaria. This should be no surprise: if a drug is targeting or depending on some metabolic pathway in the disease organism, the most straightforward adaptation of the organism would be to partially disable or work around that metabolic pathway. This type of metabolic incapacitation is also seen in the mutational response of bacteria to antibiotics. Behe’s conceptual error here is to imply that all mutational changes are like this. That is not true. In my July letter [“STAN 3”] I described several examples of bacteria acquiring antibiotic resistance without losing viability in an antibiotic-free environment [4-7], along with cases other than antiobiotic resistance where mutations increased fitness in a new environment while maintaining competitiveness in the old environment [8,9].
If the challenge that the microorganism faces is the need to expand use of a new food supply (as opposed to resist a drug trying to kill the microorganism), the adaptive response will tend to enhance metabolic pathways, rather than shut them down. The nylon bug is one example, and there are many others. We have previously discussed the experiments of Barry Hall’s team, where several separate point mutations produced a three-part system which enabled a microbe to recover the ability to metabolize lactose [10].
You have objected that even this impressive novel system merely involved “random changes which ‘tinkered’ with a pre-existing system.” But that is exactly the way evolution works! It does not create new genes out of thin air. New genes are fashioned by mutations from existing genes or their duplicates. “Since there are hardly any true novelties in evolution, every gene is a modification of an older gene. With the recent avalanche of DNA sequence data, a surprising number of unexpected similarities among proteins not previously known to be related to each other have been revealed.” [11]
These shared functional and nonfunctional DNA sequences help us to trace the common lineages of diverse organisms. Considering the striking physical variations among living things, it was a surprise for some scientists in the late twentieth century to discover the degree of commonality among genotypes. This was a key factor in changing Michael Denton’s mind on the viability of evolutionary theory.
Denton’s 1986 critique of evolution, Evolution: A Theory in Crisis, provided a key initial impetus for the Intelligent Design movement. Philip Johnson’s Darwin on Trial (1991)is largely a recitation of Evolution: A Theory in Crisis, and Behe has acknowledged that this book also set him thinking in terms of Intelligent Design [12]. Denton’s work had special credibility, since he was one of the few modern evolution skeptics with depth knowledge in the field who did not have a religious axe to grind. I have noted earlier (May, 2008) in our correspondence that Denton’s treatment of cytochrome-c variations, which was a core portion of his critique of evolution, was fundamentally mistaken. The other arguments in Evolution: A Theory in Crisis have also been refuted.
It is a tribute to Denton’s honesty that 12 years later he could publish what amounts to a recantation of his former skepticism towards evolution [13] – – the following quote is from his Nature’s Destiny (1998), where he accepts common descent and evolution, while calling attention to the fine-tuning of the laws of physics and chemistry which make life possible in our universe [14]:
So the sharp discontinuities, referred to above, between different organs and adaptations and different kinds of organisms, which have been the bedrock of antievolutionary arguments for the past century, have now greatly diminished at the DNA level. Organisms which seem very different at a morphological level can be very close together at the DNA level.
Thus, the changes in genotype required for the evolution of new life-forms are less formidable than Denton had originally surmised from just looking at the large differences in phenotype. The Intelligent Design theorists who lauded his first book have shown less diligence than Denton in coming to grips with the recent advances in biological knowledge.
(3) Further Critiques of Edge of Evolution
Since various scientists have critiqued Edge of Evolution in detail, I will not re-state all the arguments. You can use Google to search out reviews pro and con. In the Wikipedia entry on Edge of Evolution, there are many references, including Behe’s replies to critics. Below I have listed a series of brief critiques. Their scientific content seems correct, although numerous nasty personal comments regarding Behe and Intelligent Design theorists detract from the quality of these reviews.
(A) http://scienceblogs.com/pharyngula/2007/06/behes_edge_of_evolution_part_i.php
Professor P. Z. Myers of the University of Minnesota notes Behe’s confusion of probability of obtaining one particular pair of mutations with the probability of obtaining any pair of mutations. Nick Matzke likewise does the math on this [100].
(B) http://scienceblogs.com/pharyngula/2007/06/another_nail_driven_into_poor.php
Myers recaps Ken Miller’s review of Edge, focusing again mainly on confusion of probabilities.
(C) http://scienceblogs.com/pharyngula/2007/06/behes_the_edge_of_evolution_pa.php
Myers addresses specific issues of motifs of amino acids, debunking Behe’s all-or-nothing approach to protein binding.
(D) http://scienceblogs.com/pharyngula/2007/06/segmentation_genes_evolved_und.php
Myers critiques Behe’s treatment of mathematical modeling antecedents to insect segmentation.
(E) http://pandasthumb.org/archives/2008/04/behe-versus-rib.html
Ian Musgrave notes that Behe artificially limits the possibilities in evolution of protein bindings sites to multiple, simultaneous mutations in a specific sequence. However, the likely field of genetic modifications leading to new binding sites is far richer than that. Thus, it’s not as hard to develop a new binding site as Behe claims. Further, Behe ignores the evidence that most of the protein binding motifs in today’s mammals were likely originally evolved hundreds of millions of years ago in teeming single-celled organisms, and thus are now available to today’s organisms with only minor modifications needed. This addresses the problem that small breeding populations of higher animals may not provide enough scope for mutations to produce many novel protein binding sites.
(F) http://scienceblogs.com/goodmath/2007/05/behes_dreadful_new_book_a_revi_1.php
Mark Chu-Carroll analyzes some of Behe’s mathematical assumptions on fitness landscapes, etc. A fitness landscape with only two dimensions, with fitness itself being the third dimension may well have substantial, free-standing individual fitness peaks. If the genotype of a species marches by tiny steps (via random mutations and natural selection) up such a peak, then (Behe claims) the species will be “trapped” at that local optimum and unable to evolve further.
Behe implicitly assumes: (1) the genetic landscape is low-dimensional, hence will be characterized by isolated peaks separated by deep, wide, impassible valleys; (2) evolution can only proceed by tiny steps, and (3) the fitness landscape for a species doesn’t change with time; and thus he concludes that the prospect of getting stuck on isolated fitness maxima is a dire problem for evolution.
However, all three of these assumptions are wrong:
(1) The genetic landscape is high-dimensional – – the landscape is more like multiple crossing ridges than isolated peaks, so there is usually some means for a species to continue to change. These extra dimensions can include extra components of the organism’s ecological niche or plasticity of its phenotype/genotype. Also, there is nothing implausible about neutral or mildly deleterious mutations helping a species gently down into and across a fitness valley, where a subsequent beneficial mutation can then help it climb up the far side of the valley onto a new ridge. Stafford [15] provides an example of limpet behavior to demonstrate that emergent behaviors, arising from a combination of two or more existing behaviors, can play an important role in crossing fitness valleys.
(2) As noted above, a small genetic change can give a big effect on the physical organism. Chu-Carroll cites the example of the panda’s “thumb”, where a tiny genetic change leads to a substantial impact on the physique of the panda, producing what is in effect an extra thumb. So a species can make a flying leap across an apparently wide fitness valley.
(3) Finally, fitness landscapes do not remain frozen in time – – food sources and predators appear and disappear, so what worked a thousand generations ago may not work now. For instance, the widespread use of pesticides in the past few decades has led to the appearance of insecticide-resistant bugs in the wild.
(4) Status of Irreducible Complexity
In Edge, Behe continues to hold to his views on irreducible complexity which he presented in 1996 in Darwin’s Black Box. These views have been challenged by other scientists. Reasonable evolutionary explanations have been advanced which rebut most of Behe’s claimed examples of irreducible complexity. The degree to which an evolutionary explanation is possible depends on how much is known about the system in question – – if an evolutionary transition is relatively recent (say within 10 million years), and the genomes of related organisms are available to enable an estimate of what an ancestral gene looked like, there is a higher probability that scientists can discern the genetic steps involved.
At one extreme of the knowledge spectrum would be Barry Hall’s lac bug, mentioned above. Here, since it happened in laboratory organisms, we have firm knowledge about the changes to the DNA which enabled restoration of lactose metabolism. Ken Miller [16] points out that the sum of these mutations meets Behe’s criterion for meets the criteria for irreducible complexity, defined by Behe as “A single system composed of several well-matched, interacting parts that contribute to the basic function of the system, wherein the removal of any one of the parts causes the system to effectively cease functioning” (Darwin’s Black Box, p. 39). Thus, this is an example of random mutations and natural selection producing an irreducibly complex system, something Behe claims cannot happen.
Middling knowledge of possibly evolutionary pathways is available when there are living descendants of various common ancestors. For instance, Forrest and Gross [18] note the increasing complexity of the blood clotting cascade in amphioxus (a primitive cephalochordate), modern jawless fishes, modern jawed fishes, and land vertebrates. These animals are current representatives of the evolutionary line of chordates. The increasing complexity of these clotting systems, together with preserved common features, are consistent with their evolutionary development, and also show that the full complexity of today’s mammalian clotting cascade is not necessary in order to have clotting functionality. This does not necessarily prove that today’s clotting systems evolved, since it is impossible to prove anything about the past to a determined skeptic; there are folks who cannot be persuaded that the Apollo moon landings were not faked. However, it meets a requirement for internal consistency of evolutionary theory.
Biologists have likewise devised step-by-step scenarios for the buildup of other complex systems, such as the Krebs cycle and the cytochrome c oxidase protein pump. In further examples of middling knowledge, Long et al. [19] note some 22 modern genes whose step-by-step evolution from earlier genes has been detailed. Here, a comparison of genes of putatively related modern organisms is used to deduce what the ancestral gene was, and then known mutational mechanisms such as duplication, insertion, frameshifts, and point substitutions are invoked to explain exactly how the modern gene was formed from the ancestral gene. This answers Behe’s complaint that evolutionists are unable to propose the actual molecular steps involved in making new genes or functions.
A case where we have very little knowledge of how a feature evolved is the bacterial flagellum. There is little help available from comparative genomics or fossils to reconstruct the state of a bacterial genome shortly before and shortly after the emergence of the flagellum. The flagellum likely first appeared hundreds of millions of years ago. Many additional mutations have occurred since then, while maintaining the basic functionality of the flagellum. This would disguise the route of origination of the flagellum. Therefore, we should not expect biologists to be able to provide a detailed step-by-step story of its genetic evolution. They have offered various evolutionary scenarios, but these are necessarily vague and generalized. Nevertheless, scientists have pointed out a number of suggestive features. One feature is that there are thousands of different types of flagellar systems, as determined from recent genome sequences. Some of these function without all the parts associated with the standard E. coli flagellum, so “the flagellum” is not in fact irreducible or even unique [20]:
Some bacterial flagella function without the L- and P-rings. In experiments with various bacteria, some components (e.g. FliH, FliD (cap), and the muramidase domain of FlgJ) have been found helpful but not absolutely essential (Matzke 2003). One third of the 497 amino acids of flagellin have been cut out without harming its function (Kuwajima 1988). Furthermore, many bacteria have additional proteins that are required for their own flagella but that are not required in the “standard” well-studied flagellum found in E. coli. Different bacteria have different numbers of flagellar proteins (in Helicobacter pylori, for example, only thirty-three proteins are necessary to produce a working flagellum), so Behe’s favorite example of irreducibility seems actually to exhibit quite a bit of variability in terms of numbers of required parts (Ussery 1999).
All these bacterial flagella share a common set of proteins. Pallen and Matske [21] note that of some 42 proteins in E. coli or S. typhimurium flagella, about half (23) seem to be universally necessary. Of these 23 proteins, all but 2 have homologs in other (non-flagellar) systems. These facts are generally consistent with common ancestry. The BioLogos site has a good introductory discussion of the evolution of the flagellum and other complex features [97].
(5) Behe and Intelligent Design
Many critics of Behe are bewildered at his rejection of evolution yet acceptance of common ancestry. Behe writes in Edge of Evolution, “Both humans and chimps have a broken copy of a gene that in other mammals helps make vitamin C….It’s hard to imagine how there could be stronger evidence for common ancestry of chimps and humans“ (pp. 71-72), and “More compelling evidence for the shared ancestry of humans and other primates comes from .. a broken hemoglobin gene” (p. 71).
In Darwin’s Black Box Behe had proposed that in some original single-celled ancestor a Designer had placed an enormously complex genome, whose potentials got gradually unpacked over the next billion or more years as various life-forms split out in family lines. This notion was properly rejected by the scientific community, since genes that aren’t expressed for millions of years will not be preserved for future expression but will rather get lost, and since today’s genomes show no trace of being all descended from some initial super-genome. However, in Darwin’s Black Box Behe had the courage to put forth some sort of hypothesis to account for the diverse, complex forms of today’s genomes. In Edge, he snipes at Darwinism but offers nothing in the way of a testable alternative hypothesis to explain how today’s genomes came about. Thus, like most Intelligent Design writings, this book has little scientific value.
Behe claims, “Intelligent design is quite compatible with the view that the universe operates by unbroken natural law, with the design of life perhaps packed into its initial set-up.” Some progressive (old-earth) creationists believe in numerous acts of miraculous special creation, e.g. some supernatural tweak of DNA sequences, every few millennia down through the ages, to explain the appearance of each new family of organisms. Most of his critics are not aware that Behe rejects that position. But is he still holding to his earlier initial-super-genome hypothesis? Or has he become a plain theistic evolutionist? I cannot tell.
Although Behe’s objections to evolution do not stand, his treatment of the anthropic tuning of the universe is insightful. It seems clear that organic life-forms could not exist if the laws and physical constants in the universe were not tuned exactly the way they are. Some skeptics dismiss this as mere coincidence (“if the universe did not permit life, we wouldn’t be here to observe it”); more thoughtful skeptics argue that there may be an infinite number of universes in existence, with infinite variations in physical properties, so it should be no surprise that at least one universe (ours) has the properties needed to sustain life. Behe points out how the infinite multiverse alternative logically leads to such a bizarre set of possibilities of illusory observations that you would have no reason to trust your own thinking. A truly infinite multiverse, comprehending all possibilities, may well contain “freak” observers whose brains just popped into existence containing thoughts which do not correspond to any reality; and you cannot know if you are not one of those observers [23]:
What you are at this very moment “thinking” – as well as any detailed memories you have of the past, no matter how seemingly realistic, including memories of what you think you know about “nature” – could be due to a random collocation of matter that just popped into existence. The very concepts of “gravity,” “protons,” “stars” – all you think you know about nature – could be just the pitiful delusion of a freak observer. Reality may be utterly different. Such is the intellectually toxic bequest of the infinite multiverse hypothesis.
A point of view which removes any confidence that we are observing a real universe seems less conducive to scientific inquiry than the more traditional view that there is a real world which we can observe and ponder in some valid fashion.
*****************************************************************
JOHN SANFORD’S GENETIC ENTROPY AND THE MYSTERY OF THE GENOME
The errors in Genetic Entropy [24]are so pervasive that it might take a whole new book to fully expose them [93]. I’ll break it down to the topics listed below:
(1) Kimura’s Distribution of Mutations
(2) Evidence for Beneficial Mutations
(3) Gene Duplication
(4) Natural Selection: What Sanford Claims
(5) Natural Selection: What Studies Show
(6) Evidence for Genomic Deterioration
(7) Synergistic Epistasis and Other Theoretical Considerations
(8) Genetic Entropy and the Mystery of John Sanford
These topics deal with the two broad areas of random mutations and natural selection. These are the twin pillars of the neo-Darwinian synthesis, which Sanford refers to as the Primary Axiom.
(1) Kimura’s Distribution of Mutations
Sanford makes unsubstantiated claims that the number of beneficial mutations is insufficient to account for the increase in genomic complexity demanded by evolutionary theory in going from single-celled organisms to today’s mammals, or to compensate for the effects of deleterious mutations. He attempts to justify these claims in two main ways: (a) by appealing to a graph by Motoo Kimura, and (b) by citing some bacterial studies which show low rates of beneficial mutations. Kimura’s plot is discussed in this section, and the bacterial studies are treated in the following section.
Sanford presents a graph (Figure 3c), “modified and expanded” from the work of Motoo Kimura [25], which Sanford claims “very nearly represents the true distribution of mutations.“ The x-axis represents the severity of the effect of the mutations, and the vertical distance denotes the relative number of mutations with a given effect.
The curve for deleterious mutations is in the upper left quadrant. It starts near the x-axis at the extreme left (negative values of mutation effect), and rises steeply as it approaches the y-axis. It appears to asymptote to both the x- and the y-axes. It indicates that most mutations are harmful, and that mutations of small effect are more common. Sanford shows a zone of “near-neutrality” on either side of the y-axis. This is where the effect of a mutation is so subtle that it cannot be effectively selected for or against. Mutations in this zone can be regarded as “near-neutral” or “effectively neutral.” Kimura has shown that the width of this zone of near-neutrality gets smaller as the size of the effective breeding population, Ne, gets larger.
Sanford (pp.22-23) professes puzzlement over Kimura’s omission of beneficial mutations:
In Kimura’s figure, he does not show any mutations to the right of zero – i.e. there are zero beneficial mutations shown. He obviously considered beneficial mutations so rare as to be outside of consideration….…Given the pivotal role beneficial mutations play in all evolutionary scenarios, I was puzzled as to why Kimura did not represent them in any way in his graph.
Sanford notes, fairly enough, that beneficial mutations are much less common than deleterious mutations, and so he draws a little wedge-shaped curve on the right-hand side of the graph (Figure 3d) to represent beneficial mutations on the same scale.
In this exercise, Sanford (p.24) is “shocked” to find that that very few beneficial mutations are of large enough effect to be selected for:
What is most interesting about this figure (it came as a shock to me) is to realize that essentially the entire range of all hypothetical beneficial mutations falls within Kimura’s “effectively neutral” zone. That means that essentially all beneficial mutations (to the extent that they actually happen), must be “un-selectable.” So selection could never favor any such beneficial mutations, and they would essentially all drift out of the population. No wonder that Kimura preferred not to represent the distribution of the favorable mutations!
In these passages, Sanford would have his readers believe that Kimura was so dismayed by the evolutionary implications of having only small numbers of beneficial mutations that Kimura did not dare treat the subject. That is an inexcusable misrepresentation of the facts. Kimura [26] tells us explicitly why he omitted beneficial mutations from this model, and it has nothing to do with how rare or unselectable they are:
In this formulation, we disregard beneficial mutations, and restrict our consideration only to deleterious and neutral mutations. Admittedly this is an oversimplification, but as I shall show later, a model assuming that beneficial mutations also arise at a constant rate independent of environmental changes leads to unrealistic results.
The “unrealistic results” that Kimura notes of plugging beneficial mutations into his model are that “the rate of evolution can become enormously high in a very large population” (i.e. beneficial mutations would become fixed at high rates), which is not an effect that is normally observed in reality. So the reason Kimura omitted beneficial mutations is not that they have too little effect (as Sanford implies), but that in his model they would have too much effect. This is just an artifact of his model (which, like all models, is a simplification of the real situation), not a statement about whether beneficial mutations have an effect in the real world.
Kimura [27] acknowledges that with his model, slightly deleterious mutations can build up, but (using parameter values he considers realistic), he does not see this as a threat to most species:
Under the present model, effectively neutral, but, in fact, very slightly deleterious mutants accumulate continuously in every species. The selective disadvantage of such mutants (in terms of an individual’s survival and reproduction – i.e. in Darwinian fitness) is likely to be of the order of 10-5 or less, but with 104 loci per genome coding for various proteins and each accumulating the mutants at the rate of 10-6 per generation, the rate of loss of fitness per generation may amount of 10-7 per generation. Whether such a small rate of deterioration in fitness constitutes a threat to the survival and welfare of the species (not to the individual) is a moot point, but this can easily be taken care of by adaptive gene substitutions that must occur from time to time, say once every few hundred generations.
In other words, within his theoretical framework, the mutational load can be readily compensated by the occasional beneficial mutation.
All this is clearly spelled out by Kimura in The Neutral Theory of Molecular Evolution, which Sanford cites in his Genetic Entropy. Thus, Sanford misled his readers on Kimura’s treatment of beneficial mutations, and few readers would dig into Kimura’s writings to discover what he really thought.
***************************************************
Sanford notes that Kimura’s curve does not show any mutations with exactly zero effect, i.e. as absolutely neutral, and claims that, since (according to this curve) essentially all mutations are deleterious, and many of those deleterious mutations are too small to select against, genomes must accumulate ever-growing numbers of deleterious mutations which degrade the information of the genome. Thus (p.25), “Figure 3d vividly illustrates why mutations cannot result in a net gain of information…Everything about the true distribution of mutations argues against their possible role in forward evolution.”
Since this curve seems central to Sanford’s thesis, it bears some scrutiny. Here is what Kimura [28] says about the genesis of the left-hand (deleterious mutations) curve:
In our discussion of the relationship between the rate of evolution and selective constraint (see chapter 7), we often found it convenient to classify mutations into two distinct types, strictly neutral and definitely deleterious, and argued that the proportion of neutral mutations decreases with increasing functional constraint. In reality, there may be a continuum between these two types and the possibility cannot be excluded that the intermediate types between these two extremes are important. In other words, at the molecular level, a substantial proportion of new mutations could be very slightly deleterious as emphasized by Ohta (1973, 1974). Such mutations behave as if selectively neutral in a small population, but are unequivocally selected against in a large population….. I proposed (Kimura, 1979) a model which assumes that the selection coefficients among mutants follows a Gamma ( Γ) distribution and this was termed the model of effectively neutral mutations. This model is based on the idea that selective neutrality is the limit when the selective disadvantage becomes indefinitely small.
There is no experimental data of mutation frequency versus fitness effect plotted to verify the accuracy of this curve. It is not based on any actual data. It is merely an abstract relation, chosen for convenient mathematical manipulation and to illustrate a general point. It does not mean that Kimura really believes there are no truly neutral mutations; a cursory reading of his work shows that the concept of neutral mutations remains at its core. Nobody really knows the fitness distribution for mutations in humans, which is what Sanford is most concerned with, so it is inappropriate to draw hard conclusions from this curve.
**************************************************
One final distinction — as noted above, Sanford (p. 24) states that:
Essentially the entire range of all hypothetical beneficial mutations falls within Kimura’s “effectively neutral” zone. That means that essentially all beneficial mutations (to the extent that they actually happen), must be “un-selectable”.
The key, slippery word here is “essentially.” There is a world of difference between “rare” and “absent” as descriptors of beneficial mutations. Sanford makes the classic creationist leap from “the vast majority of mutations have little or no beneficial impact” to “effectively all mutations have little or no beneficial impact.” But that position is falsified by the many studies that show that organisms do in fact adapt to environmental changes by mutation and natural selection. Thus, in reality there is a small but non-negligible tail on the beneficial mutation distribution which extends far enough to the right on this type of plot to represent the beneficial mutations which are selected for.
(2) Evidence for Beneficial Mutations
The literature is rife with examples of helpful new mutations becoming fixed in a population which is exposed to a new environment. Since the fitness of an organism can only be defined in a particular environment, these mutations must be considered as beneficial for those populations. There is no necessity in evolutionary theory for a mutation which is helpful in the new environment to also be useful in the old environment. Nevertheless, there are examples where mutations caused an organism to be become more fit in a new environment, while remaining competitive in the original environment. A number of these examples were listed above [4-9]. These cases directly refute the notion that essentially all mutations are degenerative.
For most organisms, if you put them in a new environment so you can detect adaptive mutations, and wait some hundreds or thousands of generations with a population of over say 1000, you will find that adaptive, i.e. beneficial; mutations have occurred and become fixed. The appearance and fixation of beneficial mutations in situations like this is not scarce or tenuous; rather, it is common and reliable. Therefore, it is obviously wrong for creationists like Sanford to claim that beneficial mutations are so rare that they cannot be effective in producing adaptive changes.
Sanford (p. 24) mentions three studies to buttress his erroneous claim:
I have seen estimates of the ratio of deleterious-to-beneficial mutations which range from one thousand to one, up to one million to one. The best estimates seem to be one million to one (Gerrish and Lenski, 1998). The actual rate of beneficial mutations is so extremely low as to thwart any actual measurement (Bataillon, 2000; Elena et al.,1998).
We will examine these studies and their contexts to evaluate Sanford’s representations. First, Gerrish and Lenski’s [29] 1:1,000,000 estimate was for the ratio of beneficial to total mutations, not for beneficial to deleterious mutations. Although they do not directly state the nature of the 999,999 non-beneficial mutations, there is no reason to assume that they are all or mostly deleterious.
More importantly, consider the experimental data from which Gerrish and Lenski draw their conclusions: Lenski’s well-known long-term E. coli evolution study. His group set up twelve populations of E. coli, starting from a single clone progenitor, and let them evolve under nutrient-limited conditions. [30] This study was described in my July letter [“STAN 3”]. The effective breeding size of each population was about 3.3 x 107 [29].
One beneficial mutation in a million sounds like a very low number, but with thousands of generations and millions of individuals in the breeding population, there were many beneficial mutations which available in the Lenski experiment for natural selection to work with. Gerrish and Lenski [29] estimate that a new beneficial mutation occurred about every fifteen generations in their experimental populations. About 1% of the estimated beneficial mutations actually got fixed in the population. [31] For larger organisms, the breeding populations are typically lower, but the number of mutations per individual per generation is much higher, due to larger genomes and higher mutation rate per DNA base pair, so there are still lots of mutations available. However, for both microbes and mammals, evolution is typically a relatively slow process, so hundreds or thousands of generations may be required to effect a discernable change in the breeding population.
For twelve out of twelve populations in the Lenski study, a significant increase in fitness was observed over the first 10,000 generations. Thus, whatever was the percentage of beneficial mutations, they were numerous and effective enough to drive the evolution of each of the experimental populations to improved fitness. This completely refutes Sanford’s claim that “effectively all beneficial mutations…must be un-selectable.” Sanford had to be aware of the Lenski long-term E. coli study, since it was the basis of Gerrish and Lenski’s estimates of beneficial mutations, yet he withheld that information from his readers and made statements about beneficial mutations that are obviously false.
The 1998 Elena et al. study that Sanford cites also comes from Lenski’s group. Elena et al. [32] took an E. coli cell from one of the populations which had been evolved for 10,000 generations, and constructed 226 artificial mutants. Each mutant contained a single random insertion of one of three transposons. Each of these transposons encoded resistance to one of three different antibiotics. A phage was used as the delivery vehicle. These mutants were assayed for fitness relative to a common competitor. None of the mutations had a significant positive effect on fitness in their standard glucose-limited environment, whereas 80% had a significant negative effect (average 3% fitness reduction).
It is crucial to note that the starting cell had already been heavily adapted to the particular test environment for 10,000 generations. By this point, the rate of fitness increase in the population had slowed to a crawl [33]. This means that this population was so well adapted to its environment that the millions of naturally-occurring mutations in the population were conferring little further benefit. Therefore we would expect that that these mere 226 additional mutations would show no further beneficial effects, and that they were in fact likely to push it away from its local optimum. Moreover, insertion mutations of this type are likely to block the formation of any functional gene product [34]. Sanford withholds from the reader the fact that this study of artificial mutants was derived from the Lenski long-term E. coli project, which showed unequivocally that beneficial mutations are numerous and effective enough to be naturally selected and to improve the fitness of populations.
Sanford did not mention a 2001 paper by Remold and Lenski [35], which was a follow-up study to the Elena et al. [32] paper, and which demonstrated a relatively high proportion of beneficial mutations when a different environment was considered. Out of the 226 mutants from Elena et al. [32], Remold and Lenski [35] randomly chose 9 mutants from each of the three antibiotic resistance classes. Of these 27 strains, one was eliminated due to contamination. The remaining 26 strains were evaluated for fitness in 4 different assay environments: glucose medium at 28 C and at 37 C, and maltose medium at 28 C and at 37 C.
In the original glucose nutrient medium, to which the parent cell (prior to the insertions of the single mutations) had been adapted for 10,000 generations, the mutants showed fitness within about 2% percent of the progenitor. Thus, as in the original Elena et al. [32] study, no significant beneficial effects were seen from the mutations when the bacteria were tested in the glucose medium. However, Remold and Lenski [35] saw much different results in the maltose medium. The maltose medium represents a different environment, to which the parent cell had not already been adapted. In this case, there was a much wider spread of fitness. In maltose, most of the 26 mutations were deleterious, but at least 3 (i.e. 12% of the total 26 mutations represented) were significantly beneficial.
The 2001 study by Imhof and Schlotterer [39] was also accessible to Sanford at the time. Imhof and Schlotterer estimated the rate of (fixed) beneficial mutations to be 4 x 10-9 per cell per generation for cultures of E. coli, similar to the later Kassen and Bataillon study. Again, this seems like a low number, but in evolution there are many organisms and many generations, so the net amount of beneficial mutations is appreciable and effective. It is certainly not “so extremely low as to thwart any actual measurement.”
I was unable to access the Bataillon 2000 study [36] that Sanford cites, but a more recent publication from Bataillon gives a much different perspective on beneficial mutations than the one Sanford presents. Kassen and Bataillon (2006) [5] took a wild-type Pseudomonas flourescens bacterium, and exposed it to an antibiotic. They obtained over 600 antibiotic-resistant strains, with an estimated frequency of 2.4 x 10-9 beneficial mutations per cell division. That seems like a tiny number, yet it was adequate to drive the evolution of fitter bacteria. These antibiotic-resistant strains were much fitter in the new environment than the parent wild-type bacteria, which could not survive at all in the presence of the antibiotic. Interestingly, even in the absence of antibiotic, at least 2.7% of the mutants were superior to the wild-type. This is just one of the examples we have mentioned where mutant organisms can be superior to the parent in both the new environment and in the original environment.
This Kassen and Bataillon study was not available when Sanford wrote Genetic Entropy. Neither were studies by Perfeito et al. [37] which found that 1 out of every 150 mutations were beneficial in small populations of E. coli, or by Joseph and Hall [38] who found 13% of the mutations in yeast were beneficial.
(3) Gene Duplication
Sanford repeatedly asserts that mutations, by which he seems to mean simple point substitutions or single point insertion/deletion events, do not increase net information. That is generally true for point substitutions or indels, but irrelevant. By “increase net information” I assume Sanford means “increase size of the functional genome” or “increase the number of distinct genes.” This obviously will not be accomplished by just substituting one amino acid for another at a given point.
However, there is a whole other class of mutations which are common and which do increase genomic size. These are duplications and insertions of genetic material, ranging from small chunks of DNA to complete genes and to duplication of entire genomes. As usual with major mutations, most of these duplication/insertion events will be deleterious to the organism, but a small fraction will be beneficial, and some will be effectively neutral. In my letter of July [“STAN 3”] I cited three studies showing beneficial gene duplications [9, 40, 41]. Gene duplication followed by further, normal mutations provides a clear path to increasing genomic complexity. Creationists are unable to demonstrate that this path is not viable. This rebuts their claim that natural causes are inadequate to account for the increase in genomic complexity in the evolution of vertebrates from simpler organisms.
In some cases the duplicated genes are different from the parent gene, so some variation is introduced right away [9,90,91]. I am not aware of a study which has followed the genetic path of an organism through gene duplication and a subsequent major refunctionalization, but I would not expect that to be readily observable. Evolution is a very slow process by human standards. Beneficial gene duplication is rare, and the modification of a gene to serve a new function is rare, so the odds of observing both these events for a single gene in the span of a human lifetime, in an organism that happens to be under observation, are low indeed. However, in the wild, with trillions of organisms and hundreds of millions of years, the odds lengthen for successful gene duplication with neofunctionalization. The review by Long et al. [90] cited above notes a number of inferred examples of neofunctionalization of duplicated genes, but many of these transformations spanned millions of years.
Another window into the rarity of fixed mutations which increase complexity comes from examination of changes in physical forms (phenotypes) in the fossil record [44]:
Production of man from a primitive jawless fish in half a billion years is a remarkable example of progressive evolution, but we should not forget that degeneration and extinction are much more common in evolution. Haldane (1958) calls attention to the fact that probably for every case of progressive evolution in the sense of descendants being more complex in structure and behavior than their ancestors, there have been ten of regressive evolution. The main reason that evolution as a whole appears to be progressive is simply because a species that acquired a new capacity was more likely to give rise to various descendant species than one which lost some capacities.
This balance between genetic advance and decay does not imply the absence of complexity-increasing mutations, but does suggest that they are so rare that we should not expect see them occur under human observation.
Sanford tries to dismiss gene duplication with ridicule, not logic. He makes up some examples of nonsense duplications of letters and words that are not germane to genomes. He does not seem to understand that the genome is like a recipe, not a descriptive essay. Repeating a sentence in an essay may not add any information, but duplicating an instruction in a cake recipe will give a different cake. Thus, changing a recipe segment from “Add 1 cup flour” to “Add 1 cup flour; Add 1 cup flour” can materially affect the product. A further mutation to one of the duplicate instructions could give “Add 1 cup flour; Add 1 cup pudding,” which clearly contains more information that the original “Add 1 cup flour.” Of course, we would not expect these recipe permutations to survive and be recorded and shared unless the new cakes had some attractive characteristic. This would be natural selection in action.
(4) Natural Selection: What Sanford Claims
In evolutionary theory, the variations among individuals due to differing genetic endowments can lead to differing success in survival and reproduction. Thus, the genes of the fitter individuals become more highly represented in the population.
It is common for creationists to claim (incorrectly) that there are no or effectively no beneficial mutations, or none that contribute to increasing genome complexity. However, most of them seem to accept the efficacy of natural selection. A distinctive of Sanford’s work is his claim that, even if there were significant beneficial mutations, natural selection does not have the power to implement them, or to keep deleterious mutations from accumulating.
Sanford attempts to build a case that the effectiveness of natural selection is too limited to prevent the deterioration of genomes, especially human genomes. He mentions a number of theoretical or potential constraints on selection, such as:
(a) Nature sees the whole organism, so individual nucleotides cannot be selected for or against (p. 47).
(b) There are many levels of biochemical organization standing between a nucleotide and the whole organism (p. 48).
(c) Each nucleotide and gene exists in a cluster of many other nucleotides and genes (p. 54).
(d) There is a cost to selection – – some fraction of the population must be removed each generation (p. 57).
(e) Most deleterious mutations are so subtle that they do not produce an effect which is recognizable, at least to a human observer, and thus they will be difficult to select against (p. 61).
(f) Some theorists claim that it is not possible to select for many traits simultaneously (p. 78).
(g) Selection for one trait may interfere with selection for another trait, especially if the genes for the two traits are physically linked (p. 79-82).
(h) Various types of “noise” can interfere with the heritability of a trait, e.g. when an individual’s reproductive success is affected by factors other than genetic endowment (pp. 89-99).
He notes that natural selection should work more effectively with microbes, since these tend to have simpler genomes, large breeding populations, and more direct effect of selection on the individual cell. This contrasts to mammals, which typically have large genomes, more genomic interactions, and small populations which cannot tolerate a lot of selection cost (p. 74).
These factors (a)-(h) can indeed diminish the effectiveness of natural selection to some extent. Sanford, however, makes a giant and unwarranted leap to declare that they negate the broad effectiveness of natural selection. Sanford concedes that natural selection works at some limited level, i.e. that it can shape certain gene frequencies (p.64), but claims that it cannot protect higher genomes against deterioration (p. 83):
While selection is essential for slowing down degeneration, no form of selection can actually halt it….The extinction of the human genome appears just as certain and deterministic as the extinction of the stars, the death of organisms, and the heat death of the universe.
Sanford acknowledges that selective breeding can be used to promote a particular single trait such as increased seed yield in a plant, but denies that it can halt the overall degradation of genomes due to the accumulation of deleterious mutations. This claim is the core thesis of his book.
(5) Natural Selection: What Studies Show
Stability of Microbial Genomes
If all the problems ((a)-(h) above) with natural selection were as dire as Sanford claims, even microbial populations should be subject to mutational meltdown, though perhaps at a lower rate per generation than other life-forms. Microbial life-spans can be days, rather than decades. If the buildup of deleterious mutations were a real phenomenon, it would become apparent over thousands of generations in laboratory flasks of bacteria. Indeed, asexual populations like bacteria should be even more vulnerable to the buildup of deleterious mutations, since they would have less opportunity to reshuffle genes to help weed out the bad ones.
If Sanford’s thesis were correct, we might expect an initial bump up in fitness as the bacteria adapted to some new conditions, followed by a reversion to a secular decline in fitness as the supposedly relentless accumulation of deleterious mutations proceeded. In fact, the Lenski long-term E. coli experiment shows the contrary: an initially rapid increase in fitness, followed by a long plateau of stable or slightly increasing fitness for over 35,000 generations [46]. This demonstrates that Sanford is wrong. We note that the populations of cells in Lenski’s flasks are not large by microbial standards – – only about 30 million each, which compares to today’s human populations in many countries.
As for organisms in the wild, I am not aware of evidence for a general decline in microbial fitness over the past decades or millennia, which represent millions of generations. The opposite trend is suggested by how hard we must work to fend off the microbes which afflict our crops and ourselves.
Mutation Accumulation Experiments With Eukaryotes
Multi-celled eukaryotes such as animals have more complex genomes and typically smaller breeding populations than bacteria. Mutation accumulation studies have been made with animals, especially with small insects which are cheap to maintain and have short generational times. In these studies, a line or lines of descent are maintained in which natural selection is eliminated. One way to accomplish this is by having a human researcher randomly select one individual or breeding pair from each generation to produce the next generation. Whatever mutations occur in each generation are retained, since there is no opportunity to weed them out.
Classic mutation accumulation (MA) experiments were done on the fruitfly Drosophila melanogaster by Mukai [46, 47] and Ohnishi [48] in the 1960s and early 1970s. In these experiments, replicated second chromosomes were allowed to accumulate mutations, sheltered from selection by being transmitted only through heterozygous males. The egg-to-adult viability declined by 1%-2% per generation. Various assumptions were employed to analyze the data from these experiments using balancer chromosomes. The results were interpreted to mean that each newborn fly (diploid genome) harbored roughly one new moderately deleterious mutation, with average effects on viability of 1-2%, or 5-10% if homozygous. This is a relatively high load of deleterious mutations, which raised questions on how populations could maintain themselves.
MA experiments are tedious and time-consuming – Mukai’s experiments involved millions of flies. For decades there was little further work in this area, and Mukai’s results (implying a high rate of mildly deleterious mutations) were accepted as truisms, applicable to organisms in general. In the late 1990’s, however, further MA studies were undertaken which gave much different results. Experiments with Drosophila by Fernandez and Lopez-Fanjul [49-51] showed deleterious mutation rates an order of magnitude lower than Mukai, with larger effects per mutation. Keightley and Caballero [52] found almost no decline in fitness over 60 generations in MA experiments with the nematode C. elegans; the estimated rate of deleterious mutations was two orders of magnitude lower than the Mukai results. Fry, et al. [53] performed MA experiments on Drosophila with a procedure similar to Mukai, but found the decline in viability and incidence of mildly deleterious mutations was much smaller. On the other hand, Bryant and Reed [54] saw rapid decline in late-life fecundity in a MA experiment with the housefly.
Keightley and Caballero [52] and Fry, et al. [53] critiqued Mukai’s procedure and analysis, noting some internal contradictions. It is of course possible that Mukai’s and Oshniki’s work was flawed and should be set aside. However, a different perspective is provided by Baer et al. [55]. They suggest that all of the above results may be correct, simply reflecting a wide variation of mutation rates from population to population. In their own experiments, Baer et al. found significant variation in mutation rates among species of nematodes and even between strains within a species.
Another study showing variations of fitness declines between similar populations comes from Lomnicki and Jasienski [56]. Two populations of flour beetles (Tribolium confusum) were maintained for 22 generations in the virtual absence of selection. The starvation resistance of adult beetles from both selection-free populations was reduced by more than 2% per generation, while neither population showed a change in larva-to-adult survivorship rates. However, in one of these populations, the beetles showed slower development and smaller sizes of females, whereas these fitness traits were not eroded in the other population.
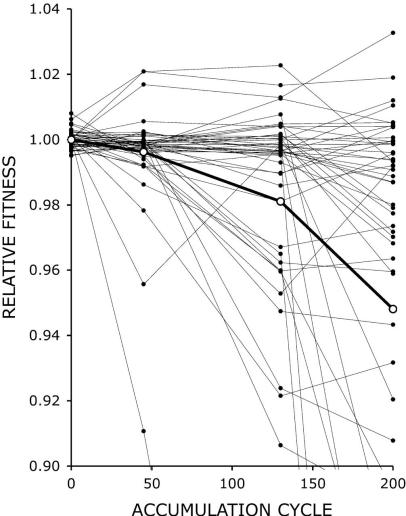
Lynch et al. [57] provide a similar perspective. They compare their detailed genomic analysis of yeast from a MA experiment by Dickinson over 4800 generations [34] with literature results for other organisms, and note significant differences among species. Drosophila seems to have an unusually high rate of deleterious mutations compared to other species.
In Dickinson’s experiment [34], 48 separate lines of yeast were maintained for 4800 generations. Fitness was assayed at the start and at three more points in the experiment. Though there was a clear trend on average towards reduced fitness, there was a wide variety in the responses of the individual lines, as shown below in Figure 1. The heavy line shows the average (mean) fitness of all 48 lines at each evaluation point. For any experiment that tries to quantitate fitness, some measurable quantity must be chosen as an estimator of fitness. Here, growth rates of samples from the lines, compared to the parent, were used to estimate fitness. Most lines showed little change in fitness, some showed dramatic decline, and a few actually showed slight fitness improvement. It seems that for any given line, a particular mutation may occur which can cause the fitness of that line to change at some atypically high or low rate. This contingency effect was seen in the Lenski long term E. coli experiment, where some particular, apparently neutral (by itself) mutation occurred early in the history in one out of twelve populations [33]. That pre-adaptive mutation enabled that population to make a jump upward in fitness many generations later.
Figure 1, taken from Dickinson [34] (Copyright © 2008 by the Genetics Society of America )
Another factor is that environmental conditions may impact the genetic fitness of a population. Shabalina et al. [58] ran an MA experiment with two populations of Drosophila kept under different conditions. The number of surviving offspring per female declined by 0.2% per generation for benign competitive conditions, and 2.0% for harsh conditions. On the other hand, Baer et al. [59] ran MA experiments with various strains of nematodes at 20 C and 25 C, and found little evidence that environment affected mutational properties.
Avila et al. [60] ran a MA experiment with Drosophila, where three large control populations were kept to compare with the MA lines. Two on the controls were kept at the normal 25 C temperature, while one was kept at 16 C. The intent with the lower temperature control was to slow down time between birth and reproduction, to produce fewer generations and thus (in theory) give even less chance for genetic change across the duration of the experiment. Surprisingly, the fitness of the second chromosomes from this control showed drastically reduced viability. This population was then brought to 25 C, and fitness recovered almost entirely after ten generations. These results show both the potential for environment to affect fitness and the power of natural selection to restore a deteriorated genome.
This survey of MA experiments reveals a rich set of phenomena. However, the following general trends are clear: in MA experiments, fitness declines at some noticeable rate (e.g. 0.1-1% per generation), whereas fitness does not decline when there is a large enough breeding population (e.g. over 2000 for eukaryotes [61] ) and conditions where differential survival or mating takes place. One can find occasional exceptions to this rule, but it holds in the vast majority of cases. This can be restated as: when natural selection is not operating, the population genome deteriorates, and when natural selection is operating, the average genome of the population does not deteriorate.
This directly contradicts Sanford’s claim that natural selection is ineffective at preventing deterioration of the genome. The factors (a)-(h) listed above are not sufficient to break the connection between gene frequencies in a population and the reproductive success of individuals carrying those genes. Natural selection is effective at weeding out deleterious mutations, despite the impacts of gene linkage, cost of selection, and noise.
This is so simple and so obvious, that we must ask: How can Sanford possibly claim what he claims, when decades of experimental studies clearly show the exact opposite? As a genetics researcher, he was certainly familiar with MA studies and their implications. This is another example of deceit in Genetic Entropy, and it is a whopper.
Fitness Recovery Experiments
The power of natural selection is further demonstrated in fitness recovery experiments, where a line where the genome has deteriorated is then bred under conditions where random mutations and natural selection can shape the gene frequencies. These conditions include a large population (to give variety of genes to select among, and to get greater opportunity for new mutations to arise) and competitive survival/breeding conditions. In the study of Avila et al. [60] noted above, a population of Drosophila had its fitness partly restored after four generations, and almost entirely restored after ten generations of breeding under more favorable conditions.
Fitness recovery experiments after a MA experiment represent a particularly challenging assignment for evolution: for these deteriorated lines, the original population genetic diversity has been lost since the breeding line in an MA experiment is typically narrowed down to just a single individual or breeding pair, and deleterious mutations have accumulated. Estes and Lynch [62] ran fitness recovery tests on MA lines of the nematode Caenorhabditis elegans, which had previously propagated as single individuals for each generation and which had suffered noticeable declines in fitness. These lines were then maintained in several large populations under competitive conditions, to allow the workings of mutations and natural selection. Progeny production and survival to maturity were assayed after 10 and after 80 recovery generations. After 10 generations, there was significant recovery of average fitness, and by 80 generations fitness recovery was nearly complete, as averaged across all the recovery populations. There was significant variation in fitness recovery from population to population, reminiscent of the variations seen among the individual lines of yeast in Dickinson’s MA study [34]. Whitlock et al. [63] cite several additional studies of fitness increase and fitness recovery in viruses and bacteria, which demonstrate the efficacy of mutations and natural selection to improve the genome.
Humans carry around 100-200 new mutations per generation [94]. That seems like a lot, but that is only about one mutation in each 15 to 30 million nucleotides. While a few of these mutations can have devastating health effects, most of these mutations have no apparent physical impact. Most of these mutations fall in regions of the genome with no known function, and many mutations in protein coding regions are “silent” mutations which do not alter the protein which is ultimately formed. Even within the coding for proteins, many of amino acids can be altered without appreciable harm to the individual [95].
The fossil record allows us to trace back some lineages for millions of years. Fossils are found in sedimentary rock layers, which record a succession of biological forms. Radioactive dating of igneous rocks above and below these sedimentary layers provide absolute dating of the sedimentary layers and thus of the fossils within them. Most species and genera go extinct and are replaced by others. However, a few have endured with little alteration for tens of millions of years, representing millions of generations. For instance, both the opossum and the crocodile have been largely unchanged for more than 60 million years. This is further evidence that genomes can endure without rapid deterioration.
As a sidebar, I am aware that creationists claim that radioactive dating of rocks is unreliable, and so these “millions of years” ages for fossils cannot be taken seriously. The creationist case against radioactive dating is based on ignorance and sloppy technique, as noted in my May letter of last year [“STAN 2”].
(6) Evidence for Genomic Deterioration
We have seen that there is overwhelming evidence that natural selection is quite able to keep genomes from deteriorating. It is even capable of enabling previously-deteriorated genomes to improve. There are many exceptions to rules in biology, so there are a few examples in the literature of fitness declining even when selection is operating in a reasonably large population. For instance, we noted earlier a case where Drosophila chromosomes deteriorated in a chilled control population [60]. We would actually expect to observe at least some cases of mutational meltdown in populations, since the fossil record indicates that over 99.9% of all species that ever existed have gone extinct. These cases do not invalid the general rule that the fitness of large populations is maintained just fine by natural selection.
In Sanford’s chapter “Is The Downward Curve Real?” I expected that he would search out a few of these exceptional studies and wave them around as though they were the norm. Instead, all he does in ten pages is present inappropriate analogies and note that the life spans of Biblical characters following Noah decline in a roughly exponential fashion. The Biblical issue is addressed below.
For all Sanford’s hand-wringing over the inescapable declines in genomes everywhere, his lack of concrete examples shows that the scientific facts are not on his side. Microbes have existed for untold millions of generations, and even small mammals like mice and rabbits which reproduce one or more times a year have existed with humans for thousands of generations in historic times, and many more thousands of generations in prehistoric times. If genomes of these rodents were declining by say 0.1% per year, then in 3000 years since 1000 BC, they should be down to 5% of their original fitness (0.999 raised to 3000 power = 0.05) So where is the evidence of super-rabbits in 1000 BC or even 1000 AD ?
Laboratory experiments with Drosophila fruitflies have been going on for a hundred years. At about two weeks per generation, that represents some 2600 generations. To my knowledge, there has not been a systemic decline in viability of the laboratory populations in this timeframe.
The only evidence of genomic deterioration that Sanford cites (in Appendix 1, pg. 175) is a study by Carlsen et al. [64], claiming this shows that “Human fertility and human sperm counts are both now dramatically declining.” This study by Carlsen was actually a “meta-study,” surveying 61 publications between 1938 and 1991. The difficulties in comparing results among different research groups across 5 decades are formidable. Other meta-studies conclude that if there has been a decline in semen quality, it is non-linear and is best explained by environmental factors, not underlying genetics [65]. The most comprehensive and self-consistent direct study on this subject was published by Andolz, et al. [66]. They report results for more than 20,000 men obtained by the same team in the same clinic in the same location (Barcelona area) for 36 years, finding no significant deterioration in sperm quality.
In general, indicators of human physical well-being have risen over the past several centuries, and have likely been fairly steady over at least the past several thousand years. Average human life spans at birth are estimated as 20-30 years for classical Greece and Rome and for medieval Britain, compared to 30-40 years in the early twentieth century and about 65 years now. This measure includes childhood death. [67] Environmental factors such as increases in nutrition and medicine over the last two hundred years likely explain the increase in the past three centuries, but the relatively steady values before 1700-1800 A.D. indicate the human race has not been deteriorating.
Although average life spans have increased, maximum life spans have remained about 105−122 calendar years throughout recorded history [68]. In Old Testament times, 70-80 years was considered a ripe old age (Psalm 90:100). This hasn’t changed a lot since then. All this contradicts Sanford’s assertion that the human genome is deteriorating.
James Crow’s Speculation on Human Genetic Deterioration
Sanford repeatedly refers (pp. 45, 65, 143, 171) to a 1997 article by James Crow [69], in which Crow speculates that human genetic fitness may be deteriorating by 1-2% per year. This sounds dire, but Crow makes it clear that the only reason he makes this suggestion is that modern hygiene and small family sizes have largely shut down natural selection among humans, such that much of the world is now running a gigantic mutation accumulation experiment. In a 2010 article [98], Michael Lynch derived a similar estimate (1% to 5%) of the expected fitness decline per generation for humans in industrial societies where improved nutrition and medical care allow the vast majority of individuals to survive to reproductive age. All would agree that under those circumstances, deleterious mutations will accumulate and fitness will decline at some rate; Crow picked 1-2% simply by analogy with Mukai’s fruitfly studies. Crow makes it clear that this fitness decline is merely a speculation on his part, not based on evidence of actual fitness decline, and that this proposed genetic deterioration only pertains to the last few centuries with greatly reduced rates of infant and childhood mortality, not to mankind’s history in general.
In his first reference to Crow (p. 45), Sanford acknowledges that Crow bases his concern on recent relaxed natural selection. However, that crucial factor is omitted in his subsequent references to Crow, which gives the impression that Crow agrees with Sanford that mutation accumulation is a general problem for the human race. In pp. 171-172, Sanford selects a whole page’s worth of quotes from Crow, focusing on the most alarming sentences (“It seems clear that for the past few centuries harmful mutations have been accumulating. …The decrease in viability from mutation accumulation is some 1 or 2% per generation…I do regard mutation accumulation as a problem. It is something like the population bomb, but it has a much longer fuse…”). Sanford’s commentary on this quote-mine is that Crow “goes on to acknowledge that humanity must now be genetically inferior to our stone-age ancestors – an amazing confession about the reality of genomic degeneration.” This implies that Crow’s work in some way supports Sanford’s contention that the human genome is inevitably declining, with or without natural selection in operation. That is grossly misleading.
Creationists and evolutionists have debated these issues in the internet. In a 2007 discussion a creationist (“AF Dave”) referred to Sanford’s citation of Crow’s work, claiming that this supported the view that natural selection is incapable of halting genomic deterioration. An evolutionist (“Dean Anderson”) then wrote to Crow, to get his views on all this. Anderson [70] posted Crow’s reply, which is reproduced in full below:
Dear Dean Anderson,
Here are my point-by-point comments on the comments of the Young Earth Creationist,
1) Harmful mutations occur every generation, but are eliminated by natural selection. Although most mutations are harmful, some are favorable and these are retained and increased by natural selection. This has been going on for billions of years.
2) I suggested in the article that in recent years, as a result of environmental improvement, the effect of natural selection was diminished. If this is correct, there are probably more harmful mutations in the population than there were a thousand or so years ago. The reason we don’t notice this is because we have greatly improved living conditions so that mutations that would have been harmful at an earlier time are much less so now.
3) If my conjecture is correct, our ancestors of a few hundred to a few thousand years ago would have had more [I think Crow meant “fewer” here] mutations than we do. This does not mean that they were stronger, fitter, or more fertile. They lived in a time of great environmental stress and would have been less strong, less fit, and less fertile than we are, thanks to the fact that our life is a lot easier than that of our ancestors.
4) My comments had to do with only the recent past (a few thousand years). In the long run, harmful mutations are eliminated by natural selection. Both mutations and natural selection have been going on since life began, billions of years ago.
5) My work (and my conjecture) offer no support for the Genesis account.
They are entirely consistent with the neo-Darwinian theory.
I hope this is useful. I would welcome your comments.
Sincerely, James F. Crow
This reply speaks for itself.
(7) Synergistic Epistasis and Other Theoretical Considerations
I know scientists who are world-class modelers of physical phenomena. They have a saying [92]: “All models are wrong, some models are useful.” Much of the confusion in Sanford’s book is due to his failure to distinguish models from reality. He siezes on the predictions of oversimplified models when they suit his case, and ignores the fact that these models obviously do not represent the real world.
Effects of Synergistic Epistasis
There is general agreement among geneticists that a simple model of mutations, with high rate of deleterious mutations operating independently, would predict that a population’s fitness and genome will deteriorate fairly rapidly. The cost of eliminating each mutation would be too high for the population to bear.
There is also general agreement that if mutations interact such that several mutations together decrease fitness more than predicted by the sum of their individual effects, then the genome will not deteriorate [69,71,72] The effect is that individuals with say four or five mutations have a much higher probability of dying young or otherwise producing few offspring, compared to the individuals with just one or two mutations. This reduces the cost to the population of eliminating the bad mutations. This is known as “synergistic epistasis”. The opposite trend is “antagonistic epistasis”, where just one mutation can have a large effect, and additional mutations have diminishing effects.
Crow [69] performed calculations regarding this issue, using realistic numbers for the Drosophila fruitfly, whose genetics have been studied for decades. He assumed an average mutation rate of one per zygote per generation, which is consistent with Mukai’s results. In any population with random mutations, some individuals will have more mutations than others. In Crow’s initial model, the 10% of the population with the highest number of mutations is selectively eliminated. He found that this “truncation selection” was very effective in eliminating mutations, at an acceptable cost.
However efficient it may be, this strict truncation with a hard limit (e.g. 10% of the population) is not realistic, as Crow [69] notes: “Of course, natural selection in either flies or people does not line up individuals and remove all of those with more than a certain number of mutations. The unreality of this model kept me for many years from considering this as a way in which the population deals with a high mutation rate.” However, “quasi-truncation selection” is reasonable. Here, the probability of selective elimination increases gradually with the number of mutations. Crow found that this form of synergistic epistasis was nearly as effective as sharp truncation: “Instead of an abrupt cutoff at 10%, consider that the probability of selective elimination increases gradually over a range of numbers of mutations. This turns out to be almost as good. If the range of gradual change is two standard deviations, the process of mutation elimination is about 87% as efficient as sharp truncation.”
Evidence for Synergistic Epistasis
Thus, synergistic epistasis can help solve the problem of mutational meltdown from a theoretical view point. The next question is whether experimental data support the operation of synergistic epistasis in actual biological systems. This turns out to be a complex issue. One method for detecting epistasis (synergistic or antagonistic) is relatively straightforward [34]:
Assessment of fitness effects in lines that have accumulated multiple spontaneous mutations is time and labor intensive but is the most direct approach (MUKAI 1969). Alternative hypotheses lead to straightforward testable predictions (KOUYOS et al. 2007). Independent action, the most useful null hypothesis, should produce multiplicative effects in combination. For multiple mutations with small effects that have not been measured individually, a plot of log fitness against the number of mutations (or the time of accumulation) should yield a straight line. Deviations from linearity indicate epistatic interaction and provide information about the nature of those interactions; negative curvature indicates synergistic epistasis while positive curvature reveals antagonistic or “diminishing returns” interactions.
However, not all studies use this approach. Some studies show synergistic epistasis and some do not. Whitlock and Bourget [73] and Avila, et al. [60] find patterns of synergistic epistasis in MA experiments with Drosophila. Dickinson [34] (see Figure 1 above, and his Figure 2 for clear deviation from linearity) saw the same trend with yeast. On the other hand, Elena and Lenksi [74] saw no evidence of epistasis when they produced insertion mutations in E. Coli, and neither did Peters and Keightley when they induced mutations in different lines of C. elegans using ethyl methanesulfonate [75].
An insightful paper by Sanjuan and Elena [76] brought understanding to this area. They reviewed 21 previous studies which looked for epistasis in fitness traits. They found a general trend of increasing synergistic epistasis with genomic complexity, running from viruses (which have very simple genomes), through bacteria and unicellular and multi-cellular eukaryotes:
Among viruses, four of six studies found antagonistic epistasis, one was inconclusive, and one found weak synergistic epistasis. In bacteria, one study found antagonism, whereas another detected no average deviation in either sense. Among unicellular eukaryotes, one-half of the studies concluded that there was no average epistasis, whereas the other half found some synergism. Finally, among pluricellular eukaryotes, seven of nine studies detected variable degrees of synergism in at least some fitness-related traits, one study reported antagonism, and one was inconclusive. Hence, despite the existence of some disparate cases, we found that most studies reporting antagonistic epistasis corresponded to organisms with simple genomes, whereas as complexity increased, cases of synergistic epistasis became more frequent (Kendall’s τb = −0.5691; 17 df; P = 0.001).
Sanjuan and Nebot [77] show that this pattern of increased synergistic epistasis for more complex genomes is consistent with network theory. For instance, a complex genome with redundancy may sustain one or more hits before a key function finally gets disabled, whereas a simple genome would get knocked out with one hit:
The study of genetic interactions (epistasis) is central to the understanding of genome organization and evolution. A general correlation between epistasis and genomic complexity has been recently shown, such that in simpler genomes epistasis is antagonistic on average (mutational effects tend to cancel each other out), whereas a transition towards synergistic epistasis occurs in more complex genomes (mutational effects strengthen each other). Here, we use a simple network model to identify basic features explaining this correlation. We show that, in small networks with multifunctional nodes, lack of redundancy, and absence of alternative pathways, epistasis is antagonistic on average. In contrast, lack of multi-functionality, high connectivity, and redundancy favor synergistic epistasis. Moreover, we confirm the previous finding that epistasis is a covariate of mutational robustness: in less robust networks it tends to be antagonistic whereas in more robust networks it tends to be synergistic. We argue that network features associated with antagonistic epistasis are typically found in simple genomes, such as those of viruses and bacteria, whereas the features associated with synergistic epistasis are more extensively exploited by higher eukaryotes.
Dickinson [34] discusses how different experimental approaches may allow or preclude the observation of synergistic epistasis. He notes that studies involving artificial mutations, especially insertions, may obscure synergistic epistasis which would otherwise appear if natural mutations were allowed to accumulate. Peck and Waxman [78] also note that experimental designs which are not naturalistic may erroneously fail to detect synergistic epistasis. They further observe that competition within small groups can readily lead to synergistic epistasis.
Kryukov et al. [79] found that synergistic epistasis is consistent with the large amount of poorly-conserved (thus nearly-neutral) non-coding regions in primate genomes. Many mutations in these regions had selection coefficient values approaching 1/Ne, where Ne is an effective population size (e.g. 10,000 for ancient humans). Malmberg and Mauricio [80] found evidence for epistatic interactions in quantitative trait locus mapping using molecular markers.
Besides genetic complexity, the rate of deleterious mutations may play a role in whether a species requires synergistic epistasis for long-term survival. For instance, many studies with the Drosophila fruitfly show a high rate of mildly deleterious mutations, but these studies also tend to show a cumulative effect of mutations that is indicative of synergistic epistasis.
On the other hand, the nematode C. elegans shows a low rate of deleterious mutations, and its genome is so robust that it can sustain the silencing of over 90% of its genes without apparent harm [77]. A study by Peters and Keightley [81] failed to detect synergistic epistasis in C. elegans. This species may not need it to maintain genetic integrity, because of its large breeding populations and low rate of fitness-altering mutations. Is possible, though, that the experimental design of this study was not well-suited for detecting synergistic epistasis [34].
Sanford’s Treatment of Synergistic Epistasis
Sanford recognizes that synergistic epistasis of deleterious mutations undermines his picture of inevitable mutational meltdown, so he attempts to discredit it. He responds first by ridicule, suggesting (page 110) that the term “synergistic epistasis” is meaningless:
It is a sophisticated-sounding expression, signifying nothing. It has all the appearance of a deliberate obfuscation. Literally translated, synergistic epistasis means “interactive interaction”.
This is knowing misrepresentation by Sanford. Further down that same page (110) he shows that he understands perfectly well what the term means: “it means that mutations interact such that several mutations cause more damage collectively than would be predicted by their individual effects.” By Sanford’s own words, synergistic epistasis is in fact a meaningful term, as contrasted with antagonistic epistasis or lack of epistasis.
Sanford addresses the experimental evidence for synergistic epistasis with a single sentence on page 110: “At least one paper provides experimental evidence that the concept is not valid (Elena and Lenski, 1997).” This statement is formally true, but grossly misleading for the general reader. Sanford claims to be most concerned about the genomes of higher organisms like animals and humans, yet he ignores the studies which show synergistic epistasis in multi-cellular eukaryotes and cites only a single study [74] on the bacteria E. coli.
We have already noted that prokaryotes like bacteria are likely to show no epistasis. Further, this particular study used artificial insertion mutations rather than random natural mutations. Dickinson [34] has pointed out that these insertion mutations are unlikely to show epistasis. So again Sanford has cherry-picked one study which is largely inappropriate yet shows the results he wants, and ignored the body of evidence that points in the opposite direction.
Immediately following his misleading treatment of synergistic epistasis, (ironically) Sanford turns around (page 110) and accuses the scientific establishment of deception:
But now, when genetic interactions can be used to obfuscate the problem of error catastrophe, the concept is conveniently trotted out and used in an extremely diffuse and vague manner – like a smoke screen. But let’s look through the smoke. If multiple mutations really do create damage in a non-linear and escalating manner, then error catastrophe would happen much sooner and populations would spiral out of control much faster – into mutational meltdown. We would already be extinct! Once again we are looking at conceptual “sleight of hand,” which clearly does not apply to the real world, and which is only aimed at propping up the Primary Axiom.
This statement errs on at least two counts. First, there has been a lively, open, and on-going discussion in the scientific community as to the occurrence and effects of synergistic epistasis, as evidenced by the sometimes-conflicting publications discussed above. Crow’s calculations demonstrating how synergistic epistasis can reduce mutational load were published in the open literature [69], subject to review before publication and critique afterward. If his calculations were in error, we would know it by now – – researchers can make a name for themselves by correcting errors by other authors.
Second, Sanford misrepresents how synergistic epistasis works. “Multiple mutations” do not occur to the same extent in all members of a population. In populations there will be some individuals who have more mild deleterious mutations than others; synergistic epistasis will tend to remove a greater fraction of those individuals, which will purge the gene pool of deleterious mutations at a lower selection cost. Only in cases where natural selection is prevented from effectively working (e.g. in mutation accumulation experiments or in very small populations) would synergistic epistasis be problematic and contribute to a mutational meltdown, but those populations would likely be in trouble anyway.
Other Issues in Population Genetics
As one moves further from simplistic models and towards the real world, additional complexities appear in population genetics. One such factor, not treated in any depth by Sanford, is sexual selection. For instance, healthy males may have more mating success than unhealthy males, even if both survive to mating age. Whitlock and Agrawal [82] show how this can act to reduce the frequency of deleterious mutations. Experiments by Hollis et al. [83] demonstrated a fitness benefit to sexual selection with Drosophila – – a deleterious gene was purged faster in populations where sexual selection was allowed to operate.
The simplest population genetics models include only deleterious mutations. Not surprisingly, these models (depending on the specific parameter values chosen) tend to show a relentless decline in species fitness, since any additional fixed mutation will degrade the genome. As noted above, this degradation is not generally observed in the laboratory or in the wild.
More realistic models include beneficial as well as deleterious mutations. The efficacy of beneficial mutations has been discussed in several places above. The exact distribution of beneficial mutations is often unknown, though some reasonable estimates can be made. Dickinson [34] argues that mutations of very small effect are as likely to be beneficial as they are to be deleterious. Charlesworth and Eyre-Walker [84] posit a class of slightly advantageous mutations, including simple back-mutations. They note that an increase in population size can lead to a temporary increase in the rate of fixed mutations when beneficial mutations are accounted for. This effect is not seen with models which include only deleterious mutations.
Whitlock et al. [63] explore the effect of population size on species survival. Large populations have several advantages. As noted above, they allow scope for natural selection to eliminate individuals with lower fitness, so fewer deleterious mutations are fixed per capita. The smaller the breeding population, the closer the situation is to a mutation accumulation experiment.
Another advantage of a large population is that it makes more new beneficial mutations available to the gene pool. Any population will have some load of deleterious mutations. In a healthy large population, the effects of new fixed deleterious mutations are balanced by new fixed beneficial mutations, so an equilibrium level of fitness is maintained. Any new fixed deleterious mutations create new opportunities for beneficial mutations; there are several classes of these compensatory mutations. However, if a population falls below a critical effective size (estimated to be in the hundreds), it can suffer mutational meltdown as new deleterious mutations are fixed at a high rate and few beneficial mutations are available to compensate.
While deleterious mutations may interact positively or negatively, it is likely that beneficial mutations show antagonistic epistasis, at least in microbial populations [96]. If a population is allowed to evolve after a change in environment or a deliberate degradation of its genome, its fitness tends to rise rapidly at first and then level out. Beneficial mutations can be lost due to competition among clones carrying different beneficial mutations, a phenomenon called the “Hill-Robertson effect” for sexual populations and “clonal interference” for asexual populations.
In Appendix 1 of Genetic Entropy, Sanford displays quotes from a number of well-known population geneticists, such as Haldane, Kimura, Muller, Kondrashov, Lynch and Crow, and attempts to show that these quotes support the notion that all genomes, especially human genomes, are inevitably deteriorating. This is his final shotgun-blast of misrepresentation to the gullible reader.
As far as I can tell, all these studies start with the simple model that includes deleterious mutations and natural selection, but excludes beneficial mutations and epistasis. Some authors make further restricting assumptions, such as asexual populations with only vertical gene transfer (Muller), no neutral mutations (Haldane), small populations (Lynch), or low modern human reproductive rate which shuts off natural selection (Muller, Crow). Such models often lead to predictions of mutational meltdown, but whenever we examine the actual, empirical data for reasonably-sized populations that extend over thousands of generations, we find no such meltdowns. Evidence of any fitness decline is rare for these populations. These data include laboratory studies of microbes, longer term historical (dozens to thousands of years) human observations of species including fruitflies and small rodents, and inferences from the fossil record.
It has been known for decades that this simple (no beneficial mutations, no epistasis) genetics model does not represent experimental reality. The recent work discussed above has helped elucidate some of the errors inherent in the simple model. The rate and efficacy of beneficial mutations is better understood, so there is no longer an excuse for not including beneficial mutations. The scope of synergistic epistasis has become clearer – it is real and effective, especially in higher organisms. Microbes have been found to horizontally exchange genetic information by a variety of mechanisms, allowing recombination and weeding out of deleterious mutations. This acts to neutralize “Muller’s ratchet.” There is a plethora of evidence that many, if not most, fixed mutations in higher organisms are effectively neutral. “Soft” selection and sexual selection can also play a role in population genetics.
All this conceptual work showing the invalidity of the simple model was accessible to Sanford, along with the observations that fitness declines do not in fact commonly occur for reasonably-sized population. However, he writes as though the simple model is valid, and quote-mines these papers for scary-sounding lines that seem to line up with his point of view.
CLOSING THOUGHTS
I invite you to compare Sanford’s approach to my treatment of the same topics in this letter. I have made an honest effort to report on the complex and the contradictory, not just pick a lone reference to prove a point.
It has been troubling to discover various instances of misrepresentation in Genetic Entropy. However, I do not believe this to be any deliberate attempt to deceive. It has been widely observed that someone who is in the grip of young earth creationism can get somewhat disconnected with reality. By all accounts, John Sanford is a sincere and godly man, a respected figure in genetics research who has accepted the price of ridicule to promote what he believes.
I’d like to thank you for the opportunity and stimulus to explore some interesting topics regarding science and faith. Searching out these references has been an educational experience for me. Last year, I investigated the young-earth creationist claims that an honest look at the physical evidence would show the earth to be thousands, not billions of years old. As I dug into each of these claims, I found them to be demonstrably false. I shared these results with you in my letter in May [“STAN 2“] of last year.
In my letter of July [“STAN 3“] this year, I examined the anti-evolutionist claims that beneficial mutations are too few and too weak to improve a species, and that mutations cannot increase information content or complexity of a genome. These claims proved also false. In this current letter, I test Behe’s claim to have demonstrated a limit to evolution, and Sanford’s claim that natural selection is ineffective in improving fitness and is not able to maintain a species against genetic deterioration. These claims, too, are false. Although much remains to be learned, evolution stands as an adequate and parsimonious explanation for the production of today’s biota from the first living cells, and for the fossil record of the intervening life-forms.
Young-earth creationism seems a failure from a scientific point of view. To the extent that it produces testable hypotheses (e.g. in Flood geology), it is readily falsified, but in most cases it doesn’t even get that far. In the area of biology, it issues mainly in complaints that evolutionary science does not yet have a demonstrated explanation of every detail. Whatever is not yet understood in terms of natural laws is hailed as evidence for supernatural intervention. This “God-of-the-gaps” approach dazzles the layman, but sets up believers for disillusionment as they find out what is known already by scientists, and as more becomes known over time. Scientists are pushing back the boundaries of our ignorance, but it is a tedious process because biological systems are so complex and multi-layered.
Bible Interpretation As The Fundamental Issue
Almost everyone who embraces young-earth creationism does so for religious or other ideological reasons, and then proceeds to ignore the mountain of facts that demonstrate its falsehood. The underlying driver is a perceived need to maintain a literal interpretation of the Biblical statements regarding creation. The motivation here is understandable: folks who have had a meaningful encounter with the God of the Bible naturally want to honor this revelation. They do not want to see the Bible as teaching error.
However, one key question is: What was the Bible intended to teach? Faith and morals? Or a definitive cosmology as well? According to II Timothy 3:15-16, the Scriptures were given to “make you wise for salvation through faith in Christ Jesus” and for “teaching, rebuking, correcting and training in righteousness,” with no mention of geology, astronomy or biology. There is a difference between Bible authors teaching versus merely assuming a recent special creation.
The original writers and readers of the Old Testament had a science of their day [85]. Folks in the ancient Middle East knew that the unmoving earth was supported on pillars, and that the stars were set in a solid firmament above the atmosphere; this firmament held back yet higher liquid waters [86]. They knew that the abode of the dead was located below the earth’s surface. They knew that living things reproduced only after their kind.
So a second key question is: Did the Genesis account work within that existing conceptual framework (not correcting it) to reveal core spiritual truths (e.g. that God is purposeful, paradoxical, relational, redemptive, the sole Creator to whom we are accountable, so we need have no superstitions regarding the natural world)? A rigorous investigation [87-89] will show that the answer to this question is “yes.”
To many believers, this seems like a shocking, sacrilegious statement. But further reflection will show that the Bible’s accommodation of then-current scientific beliefs was a wise, gracious, and appropriate policy, not an embarrassing mistake. The alternative to employing ancient cosmological concepts would have been to present creation in terms of modern knowledge, using concepts that would have confused and misled readers for the ensuing two millennia. We can’t have it both ways.
Jesus (John 5:39) warned that idolizing the text of the Scriptures could actually be an impediment to authentic relationship with him. Instead of worrying over the Bible teaching error or pretending that the ancient science (e.g. fixity of species) is correct, perhaps we should use our brains to appreciate why the Genesis account took the form it did, and to translate its inspired insights into meaningful contemporary thought-forms.
The issue of Biblical interpretation is probably the fundamental source of the differences in our points of view. This is a multi-faceted topic, which we may want to discuss in the future. [later note: see end of Grand_Canyon_Creation for more on Bible interpretation].
Best wishes,
Scott
POSTSCRIPT
John Sanford has posted a response to this essay here. Several points in his response were:
– He claims that I deny that most geneticists are concerned about current human genome deterioration. Not so. My objection to his treatment of this topic is that he did not make it sufficiently clear that their concern was generally only for modern industrial societies where natural selection is curtailed due to low birth rates and medical interventions. They do not hold that fitness has actually been declining indefinitely in pre-industrial societies, and thus it is disingenuous to cite their concerns as in any way supporting his thesis of generalized fitness decline.
– In Genetic Entropy, he characterized beneficial mutations as being so meager that essentially all of them are “un-selectable.” As I pointed out above, this is false and he must have known this to be false, e.g. (to choose just one example) because of the 1990’s Lenski experiment where all twelve populations of E. coli increased in fitness due to beneficial mutations which were obviously “selectable.” In his response here, Dr. Sanford does not acknowledge that he was wrong to characterize essentially all beneficial mutations as un-selectable, but instead tries to shift the discussion away from “beneficial” in the sense that biologists use the term, and toward changes in genome size, which is a whole different subject.
– His major claim as to the reality of genetic entropy is that some models of population genetics show unstoppable declines in population fitness due to the accumulation of small deleterious mutations. But all this shows is that we do not understand biological processes well enough to model them properly. When we go out and actually look for this supposed universal decline in fitness, it is not there, on any time scale. If a scientist’s model does not fit reality, it is his job to fix the model, not deny reality.
My full reply to his response is here.
SOME FURTHER READING: In May, 2020, Ron Garret wrote a review of Genetic Entropy which focuses on Sanford’s inappropriate treatment of “information” in the genome.
REFERENCES
(Note: The formats here are not consistent – – I mainly copied reference descriptions directly from the internet. All or nearly all the links should work if you paste them into your browser window)
[1] Michael Behe, The Edge of Evolution. New York: The Free Press, 2007.
[2] http://www.nmsr.org/nylon.htm
[3] http://en.wikipedia.org/wiki/Frameshift_mutation
[4] Johanna Björkman, Diarmaid Hughes, and Dan I. Andersson, “Virulence of antibiotic-resistant Salmonella typhimurium”, PNAS March 31, 1998 vol. 95 no. 7 3949-3953
http://www.pnas.org/content/95/7/3949.full
[5] Nature Genetics 38, 484 – 488 (2006)
Distribution of fitness effects among beneficial mutations before selection in experimental populations of bacteria Rees Kassen and Thomas Bataillon
Abstract: http://www.nature.com/ng/journal/v38/n4/abs/ng1751.html
Full Article: http://www.daimi.au.dk/~tbata/tap/ng1751.pdf ]
[6] P. Sander, B. Springer, T. Prammananan, A. Sturmfels, M. Kappler, M. Pletschette, and E. C. Bottger “Fitness Cost of Chromosomal Drug Resistance-Conferring Mutations”.
Antimicrob. Agents Chemother. 46: 1204-1211 (2002)
http://aac.asm.org/cgi/content/full/46/5/1204
[7] Naidan Luo, Sonia Pereira, Orhan Sahin, Jun Lin, Shouxiong Huang, Linda Michel, and Qijing Zhang
“Enhanced in vivo fitness of fluoroquinolone-resistant Campylobacter jejuni in the absence of antibiotic selection pressure”, PNAS January 18, 2005 vol. 102 no. 3 541-546
http://www.pnas.org/content/102/3/541.abstract
[8] Susanna K. Remold and Richard E. Lenski, “Contribution of individual random mutations to genotype-by-environment interactions in Escherichia coli” PNAS September 25, 2001 vol. 98 no. 20 11388-11393
http://www.pnas.org/content/98/20/11388.full?sid=aa94d97a-0302-417a-a669-d8684cf0be99i
[9] Celeste J. Brown, Kristy M. Todd, and R. Frank Rosenzweig, “Multiple Duplications of Yeast Hexose Transport Genes in Response to Selection in a Glucose-Limited Environment,”
Molecular Biology and Evolution, Vol 15, 931-942
http://mbe.oxfordjournals.org/cgi/reprint/15/8/931.pdf
[10] Barry Hall’s lac bug experiments are described in Kenneth Miller , Finding Darwin’s God ( New York: Perennial/Harper Collin, 1999) pp. 145-146.
[11] Gert Korthof, in http://home.planet.nl/~gkorthof/kortho36a.htm#4, citing Dan Grauer and Wen-Hsiung Li(2000) Fundamentals of Molecular Evolution, p. 264
[12] Michael Behe (Interviewee). (2003) Unlocking the Mystery of Life [Video]. USA: PBS
; cited in http://en.wikipedia.org/wiki/Behe
[13] It’s not clear how much of Denton’s 1980’s skepticism was towards evolution/common ancestry as a whole, or just towards the neo-Darwinian mechanism as a complete explanation of evolution. At any rate, Denton was recently quoted by Denis Lamoureaux in his Evolutionary Creation (Eugene, Oregon: Kipf and Stock, 2008), p.450 as follows: “There is no doubt in my mind that the process of evolution has occurred as the result of natural processes and not divine intervention even though the causal factors have not been fully identified.” In Nature’s Destiny (pp. xvii-xviii) Denton states, “It is important to emphasize at the outset that the argument presented here is entirely consistent with the basic naturalistic assumption of modern science – that the cosmos is a seamless unity which can be comprehended ultimately in its entirety by human reason and in which all phenomena, including life and evolution and the origin of man, are ultimately explicable in terms of natural processes. This is an assumption which is entirely opposed to that of the so-called “special creationist school”.
[14] Michael Denton, Nature’s Destiny: How The Laws of Biology Reveal Purpose in the Universe (New York: Free Press, 1998) p. 279.
[15] Richard Stafford, “ Exaptation and emergence as mechanisms to cross fitness valleys during evolution:an example using simulated homing behaviour ,”
Nature Precedings : hdl:10101/npre.2008.2172.1 : Posted 12 Aug 2008
[16] http://www.millerandlevine.com/km/evol/DI/AcidTest.html
[17] Michael Behe, Darwin’s Black Box (New York: Free Press, 1996), p. 59.
[18] “Biochemistry by design”
Barbara C. Forrest and Paul R. Gross, Trends in Biochemical Sciences Vol.32 No.7
http://homepages.wmich.edu/~korista/biochemistry_by_design.pdf
[19] Manyuan Long, Esther Betrán, Kevin Thornton & Wen Wang, “The origin of new genes: glimpses from the young and old,” Nature Reviews Genetics 4, 865-875 (November 2003)
[20] http://www.talkorigins.org/indexcc/CB/CB200_1.html
[21] Mark J. Pallen and Nicholas J. Matzke, “From The Origin of Species to the origin of bacterial flagella,” Nature Reviews Microbiology 4, 784-790 (October 2006); excerpt available at http://pandasthumb.org/archives/2006/09/flagellum-evolu.html ; see also http://www.talkdesign.org/faqs/flagellum.html#update
[22] Michael Behe, The Edge of Evolution. New York: The Free Press, 2007, p. 166.
[23] Michael Behe, The Edge of Evolution. New York: The Free Press, 2007, p. 226
[24] John Sanford, Genetic Entropy and the Mystery of the Genome, 2nd Ed. Lima, NY: Elim Publishing, 2005.
[25] M. Kimura, “Model of effective neutral mutations in which selective constraint is incorporated,” PNAS 76:3440-3444 1979.
[26] Motoo Kimura, The Neutral Theory of Molecular Evolution, Cambridge: Cambridge University, 1983 Press, p. 241.
[27] Motoo Kimura, The Neutral Theory of Molecular Evolution, Cambridge: Cambridge University, 1983 Press, p. 248
[28] Motoo Kimura, The Neutral Theory of Molecular Evolution, Cambridge: Cambridge University, 1983 Press, p.240-241
[29] Philip J. Gerrish and Richard E. Lenski, “The fate of competing beneficial mutations in an asexual population,” Genetica 102/103: 127–144, 1998. 127
[30] R E Lenski and M Travisano,”Dynamics of adaptation and diversification: a 10,000-generation experiment with bacterial populations,” PNAS July 19, 1994 vol. 91 no. 15 6808-6814
http://www.pnas.org/content/91/15/6808.full.pdf+html
[31] One in 10**6 mutations are significant beneficial mutations, while one in 10**8 mutations are fixed beneficial mutations, per:
Santiago F.Elena and Richard E.Lenski , “Evolution Experiments With Microorganisms: The Dynamics And Genetic Bases Of Adaptation”
Nature Reviews Genetics, 2003 Vol 4, page 457
http://www.oeb.harvard.edu/faculty/marx/Manuscripts/Elena-Lenski-2003.pdf
[32] Santiago F. Elena, Lynette Ekunwe, Neerja Hajela, Shenandoah A. Oden and Richard E. Lenski, “Distribution of Fitness Effects Caused By Random Insertion Mutations in E. Coli.” Genetica 102/103:349-358. (1998)
http://www.springerlink.com/content/r37w1hrq5l0q3832/
[33] “Slowed to a crawl” here should not be interpreted to mean that “micro” evolution had run its course and exhausted all possibilities of meaningful change in this bacteria – – In my July letter I covered some later mutations that occurred in one of the populations, resulting in the opening up of a new metabolic pathway and an upward leap in fitness, as described in:
Blount, Zachary D.; Christina Z. Borland, Richard E. Lenski (2008-06-10). “Inaugural Article: Historical contingency and the evolution of a key innovation in an experimental population of Escherichia coli”. Proceedings of the National Academy of Sciences 105 (23): 7899–7906.
[34] W. Joseph Dickinson , “Synergistic Fitness Interactions and a High Frequency of Beneficial Changes Among Mutations Accumulated Under Relaxed Selection in Saccharomyces cerevisiae,” Genetics, Vol. 178, 1571-1578, March 2008, http://www.genetics.org/cgi/content/full/178/3/1571
[35] Susanna K. Remold and Richard E. Lenski, “Contribution of individual random mutations to genotype-by-environment interactions in Escherichia coli” PNAS September 25, 2001 vol. 98 no. 20 11388-11393
http://www.pnas.org/content/98/20/11388.full?sid=aa94d97a-0302-417a-a669-d8684cf0be99
[36] T. Bataillon. “Estimation of spontaneous genome-wide mutation rate parameters: whither beneficial mutations?” Heredity. 84:497-501 (2000).
[37] Perfeito, Lilia, Lisete Fernandes, Catarina Mota and Isabel Gordo. 2007. Adaptive mutations in bacteria: High rate and small effects. Science 317: 813-815.
http://www.sciencemag.org/cgi/content/abstract/sci;317/5839/813
[38] David W. Hall, Rod Mahmoudizad, Andrew W. Hurd And Sarah B. Joseph,” Spontaneous mutations in diploid Saccharomyces cerevisiae: another thousand cell generations,” Genetics Research (2008), 90 : 229-241 http://journals.cambridge.org/action/displayAbstract?fromPage=online&aid=1919588
[39] M. Imhof And C. Schlotterer, 2001 “Fitness effects of advantageous mutations in evolving Escherichia coli populations.” Proc. Natl. Acad. Sci. USA 98:1113-1117
[40] http://toarchive.org/faqs/faq-intro-to-biology.html#mutation
“Introduction to Evolutionary Biology “
Version 2 © 1996-1997 by Chris Colby
[41] M. Richle, A. F. Bennett, and A. D. Long, “Genetic architecture of thermal adaptation in Eschericia coli,” PNAS January 16, 2001 vol. 98 no. 2 525-530
http://www.pnas.org/content/98/2/525.full
[42] – no reference –
[43] Manyuan Long, Esther Betrán, Kevin Thornton & Wen Wang, “The origin of new genes: glimpses from the young and old,” Nature Reviews Genetics 4, 865-875 (November 2003)
[44] Motoo Kimura, The Neutral Theory of Molecular Evolution, Cambridge: Cambridge University, 1983 Press, p. 61.
[45] Blount, Zachary D.; Christina Z. Borland, Richard E. Lenski (2008-06-10). “Inaugural Article: Historical contingency and the evolution of a key innovation in an experimental population of Escherichia coli”. Proceedings of the National Academy of Sciences 105 (23): 7899–7906. http://www.pnas.org/content/105/23/7899.full
[46] Mukai T(1964) Genetics 50:1–19.
[47] Mukai T, Chigusa S I, Mettler L E, Crow J F(1972) Genetics 72:335–355
[48] Ohnishi O(1977) Genetics 87:529–545
[49] Fernandez, J. & Lopez-Fanjul, C. (1997) Evolution 51 , 856–864.
[50] Avila, V. & García-Dorado, A. (2002) J. Evol. Biol. 15 , 561–566 .
[51] This 2006 reference provides later reflection on the two proceeding studies:
Victoria Ávila, David Chavarrías, Enrique Sánchez, Antonio Manrique, Carlos López-Fanjul and Aurora García-Dorado
“Increase of the Spontaneous Mutation Rate in a Long-Term Experiment With Drosophila melanogaster”
Genetics, Vol. 173, 267-277, May 2006
http://www.genetics.org/cgi/content/full/173/1/267
[52] Peter D. Keightley and Armando Caballero, “Genomic mutation rates for lifetime reproductive output and lifespan in Caenorhabditis elegans,” PNAS April 15, 1997 vol. 94 no. 8 3823-3827
http://www.pnas.org/content/94/8/3823.full
[53] James D. Fry, Peter D. Keightley, Stefanie L. Heinsohn, and Sergey V. Nuzhdin
“New estimates of the rates and effects of mildly deleterious mutation in Drosophila melanogaster”
PNAS January 19, 1999 vol. 96 no. 2 574-579
http://www.pnas.org/content/96/2/574.full
[54] Edwin H. Bryant and David H. Reed, ”Fitness Decline under Relaxed Selection in Captive Populations”. Notes – Conservation Biology. 13(3):665-669, June 1999.
[55] Charles F. Baer, Frank Shaw, Catherine Steding, Margaret Baumgartner, Alicia Hawkins, Andrew Houppert, Nicole Mason, Marissa Reed, Kevin Simonelic, Wayne Woodard, and Michael Lynch, “Comparative evolutionary genetics of spontaneous mutations affecting fitness in rhabditid nematodes,” PNAS April 19, 2005 vol. 102 no. 16 5785-5790
http://www.pnas.org/content/102/16/5785.full
[56] A Lomnicki and M Jasienski, “Does fitness erode in the absence of selection? An experimental test with Tribolium,” The Journal of Heredity 2000:91(5)
http://jhered.oxfordjournals.org/cgi/content/abstract/91/5/407
[57] Michael Lynch, Way Sung, Krystalynne Morris, Nicole Coffey, Christian R. Landry, Erik B. Dopman, W. Joseph Dickinson, Kazufusa Okamoto, Shilpa Kulkarni, Daniel L. Hartl, and W. Kelley Thomas, “A genome-wide view of the spectrum of spontaneous mutations in yeast,”
PNAS July 8, 2008 vol. 105 no. 27 9272-9277
http://www.pnas.org/content/105/27/9272.full
[58] Svetlana A. Shabalina, Lev Yu. Yampolsky, and Alexey S. Kondrashov, “Rapid decline of fitness in panmictic populations of Drosophila melanogaster maintained under relaxed natural selection,” PNAS November 25, 1997 vol. 94 no. 24 13034-13039
http://www.pnas.org/content/94/24/13034.abstract
[59] Charles F. Baer, Naomi Phillips, Dejerianne Ostrow, Arián Avalos, Dustin Blanton, Ashley Boggs, Thomas Keller, Laura Levy and Edward Mezerhane, “Cumulative Effects of Spontaneous Mutations for Fitness in Caenorhabditis: Role of Genotype, Environment and Stress,”
Genetics, Vol. 174, 1387-1395, November 2006
http://www.genetics.org/cgi/content/full/174/3/1387
[60] Victoria Ávila, David Chavarrías, Enrique Sánchez, Antonio Manrique, Carlos López-Fanjul and Aurora García-Dorado, “Increase of the Spontaneous Mutation Rate in a Long-Term Experiment With Drosophila melanogaster,” Genetics, Vol. 173, 267-277, May 2006
http://www.genetics.org/cgi/content/full/173/1/267
[61] David H. Reed,”Relationship between population size and fitness” Conservation biology 2005, vol. 19, no2, pp. 563-568 [6 page(s) (article)] (1 p.1/4)
Click to access pop%20size%20and%20fitness%20Reed%2005.pdf
[62] Suzanne Estes and Michael Lynch, “ Rapid Fitness Recovery In Mutationally Degraded Lines Of Caenorhabditis Elegans” Evolution 57(5):1022-1030. 2003
[63] M. C Whitlock, C. K. Griswold, and A. D. Peters (2003) “Compensating for the meltdown: The critical effective size of a population with deleterious and compensatory mutations”. Annales zoologici Fennici, 40, 169-183.
Click to access anzf40-169.pdf
[64] Carlsen E, Giwercman A, Keiding N, Skakkebaek NE. “Evidence for decreasing quality of semen during past 50 years”. BMJ 1992;305:609-12.
[65] James W. Crissman, Paul S. Cooke, Rex A. Hess, M. Sue Marty and Ashley B. Liberacki, “Postulated Human Sperm Count Decline May Involve Historic Elimination of Juvenile Iodine Deficiency: A New Hypothesis with Experimental Evidence in the Rat”
Toxicological Sciences 53, 400-410 (2000)
http://toxsci.oxfordjournals.org/cgi/content/full/53/2/400 ;
Marilyn F Vine, BMJ 1996;312:506 (24 February) http://www.bmj.com/cgi/content/full/312/7029/506 ;
A. Brake and W. Krause “Decreasing quality of semen” BMJ. 1992 December 12; 305(6867): 1498 . http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1884126/?page=1 .
[66] P. Andolz, M.A. Bielsa and J. Vila, “Evolution of semen quality in North-eastern Spain: a study in 22,759 infertile men over a 36 year period” Human Reproduction, Vol. 14, No. 3, 731-735, March 1999
http://humrep.oxfordjournals.org/cgi/content/full/14/3/731
[67] http://en.wikipedia.org/wiki/Life_expectancy
[68] http://en.wikipedia.org/wiki/Maximum_life_span
[69] James F. Crow,”The high spontaneous mutation rate: Is it a health risk?”
Proc. Natl. Acad. Sci. USA Vol. 94, pp. 8380-8386, August 1997
http://www.pnas.org/content/94/16/8380.full
[70] This was posted on June 27, 2007 by Dean Anderson on a thread on the Internet Infidels Discussion Board (www.iidb.org)
The link I used was: http://www.freeratio.org/showthread.php?t=211915&page=9.
[71] A.S. Kondrashov. 1995. “Contamination of the genome by very slightly deleterious mutations: Why have we not died 100 times over?” J Theor. Biol. 175:583-594.
[72] M.W. Nachman and S.L. Crowell. 2000. “Estimate of the mutation rate per nucleotide in humans.” Genetics 156:297-304.
[73] W. C. Whitlock and D. Bourguet, “Factors affecting the genetic load in Drosophila: synergistic epistasis and correlations among fitness components”. .
Evolution. 2000 Oct;54(5):1654-60
http://www.ncbi.nlm.nih.gov/sites/entrez?cmd=Retrieve&db=PubMed&list_uids=11108592
[74] S. F. Elena and R.E. Lenski, “Test of synergistic interactions among deleterious mutations in bacteria”. Nature (1997) 390:395-398
http://parasito-evolutive.snv.jussieu.fr/Jacob/lectures/papers.sex/Elena.Nature.1997.pdf
[75] Andrew D. Peters and Peter D. Keightley, “A Test for Epistasis Among Induced Mutations in Caenorhabditis elegans,” Genetics, Vol. 156, 1635-1647, December 2000
[76] Rafael Sanjuán and Santiago F. Elena, “Epistasis correlates to genomic complexity” PNAS September 26, 2006 vol. 103 no. 39 14402-14405
http://www.pnas.org/content/103/39/14402.full
[77] Rafael Sanjuán and Miguel R. Nebot,“A Network Model for the Correlation between Epistasis and Genomic Complexity,” PLoS ONE. 2008; 3(7): e2663
http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2481279
[78] Joel R. Peck & David Waxman, “Mutation and sex in a competitive world”
Nature 406, 399-404 (27 July 2000) http://www.nature.com/nature/journal/v406/n6794/full/406399a0.html
[79] Gregory V Kryukov, Steffen Schmidt and Shamil Sunyaev, “Small fitness effect of mutations in highly conserved non-coding regions” Molecular Genetics 2005 14(15):2221-2229
http://hmg.oxfordjournals.org/cgi/content/full/14/15/2221
[80] Russell L. Malmberg And Rodney Mauricio, “QTL-based evidence for the role of epistasis in evolution” Genetical Research (2005), 86:2:89-95 Cambridge University Press
http://journals.cambridge.org/action/displayAbstract?fromPage=online&aid=360631
[81] Andrew D. Peters and Peter D. Keightley, “A Test for Epistasis Among Induced Mutations in Caenorhabditis elegans “ Genetics, Vol. 156, 1635-1647, December 2000
[82] Michael C. Whitlock and Aneil F. Agrawal , “Purging the Genome with Sexual Selection: Reducing Mutation Load Through Selection on Males”
Evolution 63(3):569-582. 2009
http://www.bioone.org/doi/abs/10.1111/j.1558-5646.2008.00558.x
[83] Brian Hollis,Janna L. Fierst, and David Houle, “Sexual Selection Accelerates The Elimination Of A Deleterious Mutant In Drosophila Melanogaster “ Evolution 63(2):324-333. 2009
http://www.bioone.org/doi/abs/10.1111/j.1558-5646.2008.00551.x?journalCode=evol
[84] Jane Charlesworth and Adam Eyre-Walker, “The other side of the nearly neutral theory, evidence of slightly advantageous back-mutations” PNAS October 23, 2007 vol. 104 no. 43 16992-16997 http://www.pnas.org/content/104/43/16992.full
[85] John Walton
Ancient Near Eastern Thought and the Old Testament: Introducing the Conceptual World of the Hebrew Bible (Grand Rapids: Baker Academic, 2006), 165-167, in http://science.drvinson.net/non-concordism
[86] Paul H. Seely, “THE FIRMAMENT AND THE WATER ABOVE Part I: The Meaning of raqia in Gen 1:6-8” The Westminster Theological Journal 53 (1991) 227-40
Paul H. Seely, “The firmament and the waters above, Part II: The Meaning of ‘the water above the firmament’ in Gen. 1:6-8”
Westminster Theological Journal 54(1992) 31-46
http://www.thedivinecouncil.com/seelypt2.pdf
See also: Was the “Expanse” Overhead in Genesis 1 a Solid Dome?
[87] Paul Seely’s pioneering book here is Inerrant Wisdom: Science and Inerrancy in Biblical Perspective (1989), published by Evangelical Reform, Portland, OR. This book was recently reviewed by Peter Enns
http://peterennsonline.com/book-reviews/review-inerrant-wisdom-by-paul-seely/
Besides the two papers listed in reference [86], other Seely papers are available on-line:
http://www.asa3.org/ASA/PSCF/1997/PSCF6-97Seely.html
Click to access seely.babel.wtj.2001.pdf
Click to access PSCF9-04Seely.pdf
http://www.asa3.org/ASA/PSCF/2007/PSCF3-07Seely.pdf
http://www.asa3.org/ASA/PSCF/2007/PSCF3-07Seely2.pdf
A similar enlightened viewpoint is taken by Denis Lamoureux, e.g. in Evolutionary Creation: A Christian Approach to Evolution . Eugene, OR: Wipf and Stock, (2008).
[88] This website provides definitions of the positions and links to papers on all sides of the science/Bible issues:
http://www.asa3.org/ASA/education/origins/concordism.htm
and this site has excerpts from many authors who deal with these issues:
http://science.drvinson.net/non-concordism
“Concordism” is the view that the science in the Bible must formally agree with modern science. Young earth creation tries to accomplish this by denying the findings of modern science, while old-earth or progressive (day/age) creationism tries to accomplish this by reinterpreting Genesis 1 to find it teaching the Big Bang and geology spanning billions of years. But most old-earth creationists deny macroevolution, which forces them to posit that God supernaturally intervened many times over the ages to create new families of organisms.
[89] The following paper gives an introduction to this topic:
http://www.asa3.org/ASA/topics/Bible-Science/6-02Watts.html
“Making Sense of Genesis 1” by Rikki E. Watts
[90] Manyuan Long, Esther Betrán, Kevin Thornton & Wen Wang, “The origin of new genes: glimpses from the young and old,” Nature Reviews Genetics 4, 865-875 (November 2003)
[91] Vaishali Katj and Michael Lynch, “On the Formation of Novel Genes by Duplication in the Caenorhabditis elegans Genome,” Molecular Biology and Evolution 2006 23(5):1056-1067
http://mbe.oxfordjournals.org/cgi/content/abstract/23/5/1056
[92] Attributed to statistician George Box.
[93] When I started evaluating Genetic Entropy in 2008, the main existing scientific reviews were a pair of reviews of Genetic Entropy by Ricardo Azevedo, which appeared on Azevedo’s blog Newton’s Binomium in October, 2006: http://newtonsbinomium.blogspot.com/2006/10/review-of-mystery-of-genome-i.html and
http://newtonsbinomium.blogspot.com/2006/10/review-of-mystery-of-genome-ii.html . These reviews make a some general statements with minimal support, and sometimes simply assume the truth of evolution, which a YEC might criticize as circular reasoning. These reviews were quick, brief blog entries, based on Azevedo’s admittedly partial reading of Genetic Entropy, so perhaps we should not expect too much from them. In the absence of a prior substantive critique of Genetic Entropy, I decided to review it thoroughly here.
[94] Wellcome Trust Sanger Institute. “We Are All Mutants: Measurement Of Mutation Rate In Humans By Direct Sequencing.” ScienceDaily 1 September 2009.
http://www.sciencedaily.com/releases/2009/08/090827123210.htm
[95] Michael Lynch, “ Simple evolutionary pathways to complex proteins,” Protein Science (2005), 14:2217-2225
http://www.proteinscience.org/cgi/content/full/14/9/2217 .
[96] Sergey Kryazhimskiy, Gašper Tkačik, and Joshua B. Plotkin, “The dynamics of adaptation on correlated fitness landscapes”, Proc Natl Acad Sci U S A. 2009 November 3; 106(44): 18638–18643
“How can evolution account for the complexity of life on earth today?”
[98] Michael Lynch, “Rate, molecular spectrum, and consequences of human mutation”, PNAS January 19, 2010 vol. 107 no. 3 961-968 http://www.pnas.org/content/107/3/961.full.pdf+html
[99] Glenn Morton’s original identification of the demon is here: http://home.entouch.net/dmd/mortonsdemon.htm and reposted here: http://www.talkorigins.org/origins/postmonth/feb02.html . A cartoon version appears here: http://pandasthumb.org/archives/2009/06/mortons-demon–.html
[100] http://pandasthumb.org/archives/2007/10/full-text-of-th.html#more
[101] see e.g. Okamura et al., “Frequent appearance of novel protein-coding sequences by frameshift translation”, Genomics ,Volume 88, Issue 6, December 2006, Pages 690–697 http://www.sciencedirect.com/science/article/pii/S0888754306001807
[102] Dennis Venema, “Understanding Evolution: the Evolutionary Origins of Irreducible Complexity (Part 5)” , http://biologos.org/blog/understanding-evolution-evolutionary-origins-of-irreducible-complexity-5
This article also describes the Lenski experiment which produced that four-part mutation,along with several other lab-observed mutations which gave new functions:
Evolution Before Our Eyes: Complex Mutations in Microbes Giving New Functions
COPYRIGHT SCOTT BUCHANAN 2010
Permission is granted for reproduction for non-commercial use as long as the contents are not altered and attribution is given. Figure attributed separately.